Sodium-glucose cotransporter 2 (SGLT2) inhibitors and risk of chronic kidney disease-mineral and bone disorders in patients with type 2 diabetes mellitus and stage 1-3 chronic kidney disease
- PMID: 39993818
- PMCID: PMC11867598
- DOI: 10.1503/cmaj.240922
Sodium-glucose cotransporter 2 (SGLT2) inhibitors and risk of chronic kidney disease-mineral and bone disorders in patients with type 2 diabetes mellitus and stage 1-3 chronic kidney disease
Abstract
Background: In patients with type 2 diabetes mellitus and chronic kidney disease (CKD), sodium-glucose cotransporter 2 (SGLT2) inhibitors improve renal outcomes, but may transiently affect biochemical markers of CKD-mineral and bone disorders (CKD-MBD). We sought to evaluate the long-term risk of CKD-MBD associated with use of SGLT2 inhibitors in this patient population.
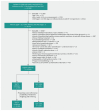
Methods: We conducted a retrospective cohort study, employing a target trial emulation framework and using electronic medical records of patients from 9 hospitals in Taiwan (2016-2023). We included adults with type 2 diabetes mellitus and stage 1-3 CKD who had newly started either an SGLT2 inhibitor or, as a comparison group, a glucagon-like peptide-1 receptor agonist (GLP-1 RA). The primary outcome was a composite of incident biochemical abnormalities (serum phosphate > 1.5 mmol/L, serum calcium < 2.1 mmol/L, serum intact parathyroid hormone [iPTH] > 6.9 pmol/L, or serum 25-hydroxyvitamin D < 49.9 nmol/L).
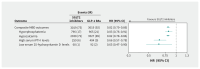
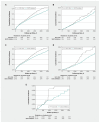
Results: The cohort included 13 379 patients receiving SGLT2 inhibitors (n = 11 920) or GLP-1 RAs (n = 1459) with a median follow-up of 3.3 years. Compared with GLP-1 RAs, SGLT2 inhibitors were associated with a lower cumulative incidence of the composite primary outcome (hazard ratio [HR] 0.82, 95% confidence interval [CI] 0.79-0.86), hyperphosphatemia (HR 0.83, 95% CI 0.76-0.91), hypocalcemia (HR 0.82, 95% CI 0.78-0.86), high serum iPTH levels (HR 0.66, 95% CI 0.57-0.78), and low serum 25-hydroxyvitamin D levels (HR 0.65, 95% CI 0.47-0.90).
Interpretation: Use of SGLT2 inhibitors was associated with a lower incidence of biochemical abnormalities related to CKD-MBD than GLP-1 RAs. These agents may be considered to reduce risk of CKD-MBD in patients with type 2 diabetes mellitus and stage 1-3 CKD.
© 2025 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests:: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
