Plain versus drug balloon and stenting in severe ischaemia of the leg (BASIL-3): open label, three arm, randomised, multicentre, phase 3 trial
- PMID: 39993822
- PMCID: PMC11848676
- DOI: 10.1136/bmj-2024-080881
Plain versus drug balloon and stenting in severe ischaemia of the leg (BASIL-3): open label, three arm, randomised, multicentre, phase 3 trial
Abstract
Objective: To determine which primary endovascular revascularisation strategy represents the most clinically effective treatment for patients with chronic limb threatening ischaemia who require endovascular femoro-popliteal, with or without infra-popliteal, revascularisation.
Design: Three arm, open label, pragmatic, multicentre, randomised, phase 3 superiority trial (BASIL-3).
Setting: 35 UK NHS vascular units.
Participants: Patients with chronic limb threatening ischaemia who required endovascular femoro-popliteal, with or without infra-popliteal, revascularisation.
Interventions: Participants were randomly assigned (1:1:1) to femoro-popliteal plain balloon angioplasty with or without bare metal stenting (PBA±BMS), drug coated balloon angioplasty with or without bare metal stenting (DCBA±BMS), or drug eluting stenting (DES) as their first revascularisation strategy.
Main outcome measures: The primary outcome was amputation free survival defined as time to first major amputation or death from any cause. Secondary outcomes included the composite components of the primary outcome, major adverse limb events, major adverse cardiac events, and other prespecified clinical and patient reported outcome measures. Serious adverse events were collected up to 30 days after the first revascularisation procedure.
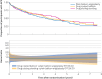
Results: Between 29 January 2016 and 31 August 2021, 481 participants were randomised (167 (35%) women, mean age 71.8 years (standard deviation 10.8)). Major amputation or death occurred in 106 of 160 (66%) participants in the PBA±BMS group, 97 of 161 (60%) in the DCBA±BMS group, and 93 of 159 (58%) in the DES group (adjusted hazard ratios: PBA±BMS v DCBA±BMS: 0.84, 97.5% confidence interval 0.61 to 1.16, P=0.22; PBA±BMS v DES: 0.83, 0.60 to 1.15, P=0.20). No differences in serious adverse events were reported between the groups.
Conclusions: Neither DCBA±BMS nor DES conferred significant clinical benefit over PBA±BMS in the femoro-popliteal segment in patients with chronic limb threatening ischaemia undergoing endovascular femoro-popliteal, with or without infra-popliteal, revascularisation.
Trial registration: ISRCTN registry ISRCTN14469736.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from UK National Institute for Health and Care Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Conte MS, Bradbury AW, Kolh P, et al. GVG Writing Group for the Joint Guidelines of the Society for Vascular Surgery (SVS), European Society for Vascular Surgery (ESVS), and World Federation of Vascular Societies (WFVS) . Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58(1S):S1-109, 109.e33. 10.1016/j.ejvs.2019.05.006 - DOI - PMC - PubMed
-
- Bradbury AW, Adam DJ, Bell J, et al. . Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess 2010;14:1-210, iii-iv. 10.3310/hta14140 - DOI - PubMed
-
- Bradbury AW, Moakes CA, Popplewell M, et al. BASIL-2 Investigators . A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet 2023;401:1798-809. 10.1016/S0140-6736(23)00462-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
