Safety, tolerability, and immunogenicity of pentavalent meningococcal MenABCWY vaccine in healthy infants: A phase 2b randomized clinical trial
- PMID: 39993937
- PMCID: PMC11853628
- DOI: 10.1080/21645515.2025.2463194
Safety, tolerability, and immunogenicity of pentavalent meningococcal MenABCWY vaccine in healthy infants: A phase 2b randomized clinical trial
Abstract
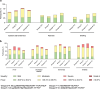
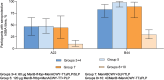
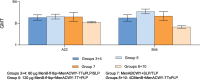
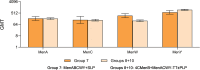
Invasive meningococcal disease is an uncommon but serious disease predominantly affecting children. This phase 2b study evaluated MenABCWY in 6-month-old infants followed by MenB-fHbp and MenABCWY in 2-month-old infants, the latter being the target age and intervention. Participants were randomized to MenABCWY, 60 µg or 120 µg MenB-fHbp+MenACWY-TT, or 4CMenB+MenACWY-TT, administered as 2 primary and 1 booster dose. The primary safety objective was to describe the safety profile of MenABCWY in participants enrolled at 2 months. Primary immunogenicity objectives were the percentage of participants achieving seroprotective serum bactericidal antibody using human complement titers. Overall, 314 and 12 participants were randomized to sentinel cohort and open-label expanded-enrollment stages, respectively. Based on 2 reports of fever requiring invasive investigations and accompanied by cerebrospinal fluid pleocytosis and 1 report arising from a previous study, the Sponsor terminated the study. Local reactions and systemic events after primary vaccination were generally mild to moderate, and tended to be higher with MenABCWY versus 4CMenB+MenACWY-TT. Immunogenicity data suggest that 1 month after vaccination 2, MenABCWY responses for MenA/C/W/Y were robust and comparable with 4CMenB+MenACWY-TT in 2-month-old participants. Immune responses for MenB test strains were higher with MenABCWY versus 4CMenB+MenACWY-TT and generally similar with 60 µg and 120 µg MenB-fHbp+MenACWY-TT or MenABCWY. Based on the limited results, the consistency of MenB immune responses with 60 µg and 120 µg MenB-fHbp suggests doses < 60 µg could be investigated to assess whether a more acceptable safety profile in conjunction with beneficial immune responses is possible in 2-month-old infants.
Keywords: Invasive meningococcal disease; MENABCWY; MenACWY-TT; bexsero; immunogenicity; nimenrix; pediatric; safety; trumenba; vaccination.
Conflict of interest statement
Erik Lamberth, Lefteris Zolotas, Islamiat Oldaipupo, James Trammel, Robert O’Niell, Paul A. Liberator, Paula Peyrani, William C. Gruber, Annaliesa S. Anderson, and Johannes Beeslaar are or were employees of Pfizer at the time of study conduct and may hold stock or stock options in the company. Federico Martinón-Torres has acted as coordinator investigator in this trial, with fees paid to his institution; has acted as principal investigator in randomized controlled trials for Ablynx, Abbot, Seqirus, Sanofi Pasteur MSD, Sanofi Pasteur, Cubist, Wyeth, Merck, Pfizer, Roche, Regeneron, Jansen, Medimmune, Novavax, Novartis, and GSK, with honoraria paid to his institution; and reports a consulting or advisory relationship with GSK Vaccines SRL, Pfizer, Sanofi Pasteur, Janssen Pharmaceuticals, MSD, and Seqirus Pty Ltd.
Figures


References
-
- Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81(4):483–498. doi:10.1016/j.jinf.2020.05.079. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
