Drop-shaped microgrooves guide unidirectional cell migration for enhanced endothelialization
- PMID: 39994203
- PMCID: PMC11850906
- DOI: 10.1038/s41467-025-57146-5
Drop-shaped microgrooves guide unidirectional cell migration for enhanced endothelialization
Abstract
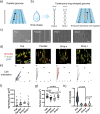
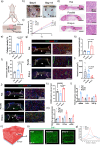
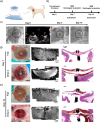
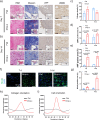
Atrial fibrillation (AF) significantly increases the risk of ischemic stroke, and in non-valvular AF, 90% of stroke-causing thrombi arise from the left atrial appendage (LAA). Percutaneous LAA occlusion using an occluder is a crucial clinical intervention. However, occluder materials could provoke thrombi, termed device-related thrombosis (DRT), leading to treatment failure. Rapid endothelialization is essential to address the DRT but the occluder's large surface area and irregular cell migration on the surface impede this process. Here, we report a continuous drop-shaped microgroove, which has a drop-shaped unit structure similar to endothelial cells. The microgrooves polarize the cytoskeleton, guiding cell unidirectional migration within the grooves, and increase cell migration efficiency. We show that drop-shaped microgrooves accelerate wound healing in a rat model, and that occluder discs with drop-shaped microgrooves promote endothelialization in a canine model. Together, our results show that integrating microgrooves with medical devices is a promising approach for addressing DRT.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.J., K.R., X.W., J.W., G.F., and L.Y. are inventors of a patent related to this work filed by Zhejiang University (2023106046243), the remaining authors declare no competing interests.
Figures



References
-
- Hart, R. G., Pearce, L. A. & Aguilar, M. I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med.146, 857–867 (2007). - PubMed
-
- Saposnik, G., Gladstone, D., Raptis, R., Zhou, L. & Hart, R. G. Atrial fibrillation in ischemic stroke. Stroke44, 99–104 (2013). - PubMed
-
- Katsanos, A. H., Kamel, H., Healey, J. S. & Hart, R. G. Stroke prevention in atrial fibrillation. Circulation142, 2371–2388 (2020). - PubMed
-
- Caliskan, E. et al. Interventional and surgical occlusion of the left atrial appendage. Nat. Rev. Cardiol.14, 727–743 (2017). - PubMed
-
- Liuzzo, G. & Patrono, C. Occluding to prevent occlusion. Eur. Heart J.42, 3224–3225 (2021). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

