Viral and immune profiles during the first wave of SARS-CoV-2 infection in hospitalized patients in Sardinia, Italy
- PMID: 39994243
- PMCID: PMC11850715
- DOI: 10.1038/s41598-025-90324-5
Viral and immune profiles during the first wave of SARS-CoV-2 infection in hospitalized patients in Sardinia, Italy
Abstract
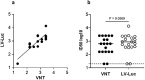
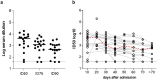
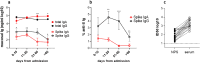
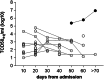
We performed a retrospective immunological analysis of the antibody response in serum and in nasopharyngeal swabs (NPS) obtained from 46 individuals infected with ancestral SARS-CoV-2 Wuhan-Hu-1 strain during the first COVID-19 wave in Cagliari (Sardinia, Italy), with a 4-month follow-up after the hospital admission. We implemented a comprehensive antibody response in serum and in mucosal samples using assays established in our laboratories. In NPS we evaluated the viral load by real time PCR, presence and kinetics of anti-Spike IgG and IgA by ELISA as well as their anti-Wuhan neutralization activity, showing induction and persistence of anti-viral immunity at the mucosal level. Neutralizing antibodies were measured in serum and NPS using a safe pseudovirus-based assay validated after comparison with a standard neutralization test using live SARS-CoV-2. We evaluated cross-neutralizing antibodies against all the major early variants of concerns (VoC) in sera. Of note, we detected a remarkable reduction of neutralizing activity against BA.1 compared to BA.2 and BA.5 Omicron subvariants, which was confirmed in sera from an analogous cohort of patients at the San Raffaele hospital in Milan, a geographically distant region of Italy, infected with the ancestral virus during the same period of time.
Keywords: Mucosal antibodies; Neutralizing antibodies; SARS-CoV-2; Sardinia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The subjects belonged to the Ospedale SS Trinità. The observational and retrospective study “COVID-IMM: Analisi della risposta IMMunologica nei sieri di pazienti COVID-19” has been approved by the ATS Sardegna Ethics Review Board (protocol 260/2020). COVID-19 Patients characterization, Biobank, Treatment response and Outcome Predictor [COVID-BioB], EC protocol number 34/int/2020, clinicalTrials.gov NCT04318366. Consent to participate: Informed consent was obtained from all participants included in the study.
Figures


References
-
- Bilancio demografico mensile [Internet]. [cited 2024 Feb 22]. Available from: https://demo.istat.it/app/?l=it&a=2020&i=D7B
-
- Impatto dell’epidemia COVID-19 sulla mortalità totale della popolazione residente periodo Gennaio-Novembre 2020 [Internet]. Available from: https://www.epicentro.iss.it/coronavirus/pdf/Rapp_Istat_Iss_gennaio-nove...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

