Safety and effectiveness of triple-drug therapy with ivermectin, diethylcarbamazine, and albendazole in reducing lymphatic filariasis prevalence and clearing circulating filarial antigens in Mombasa, Kenya
- PMID: 39994719
- PMCID: PMC11849337
- DOI: 10.1186/s40249-025-01282-z
Safety and effectiveness of triple-drug therapy with ivermectin, diethylcarbamazine, and albendazole in reducing lymphatic filariasis prevalence and clearing circulating filarial antigens in Mombasa, Kenya
Abstract
Background: In 2018, Kenya introduced triple-drug therapy with ivermectin, diethylcarbamazine, albendazole (IDA) through mass drug administration (MDA) to accelerate the elimination of lymphatic filariasis (LF). This community-based surveillance study assessed the safety and effectiveness of IDA-MDA in reducing LF-antigenemia prevalence and circulating filarial antigens (CFA) clearance among LF infected individuals.
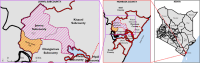
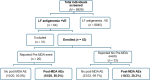
Methods: A total of 8928 residents in Mombasa, Kenya, were screened for CFA using the Filarial Test Strip: 3464 were screened in 2018 and 5464 in 2021 after two annual IDA-MDA rounds. CFA-positive individuals in 2021 were re-tested at two and four months of post-MDA for CFA-clearance rates. Adverse events (AEs) associated with IDA-MDA were monitored via door-to-door visits on days 1, 2, and 7 post-MDA to document the incidence, type and risk factors. Efficacy outcomes included post-MDA LF-antigenemia prevalence reduction after two rounds of annual MDA and CFA clearance rate. Chi-square test compared proportions, and logistic regression analysis identified AE predictors.
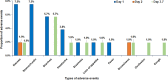
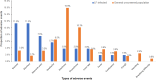
Results: LF antigenemia prevalence significantly decreased from 1.39% (n = 48) in 2018 to 0.66% (n = 36) in 2021 [P < 0.001; 95% confidence interval (CI) for difference in proportions: 0.003-0.012]. CFA clearance rates were 63.2% (12/19, 95% CI: 41.0-80.1%) at 2 months and 68.4% (13/19, 95% CI: 46.0-86.6%) at 4 months post-MDA. Among 53 CFA-positive individuals monitored, the cumulative 7-day AE incidence was 37.7% (95% CI: 25.6-51.7), higher than the general population's 27.3% (95% CI: 26.4-28.2). Common AEs included nausea (11.3%), diarrhea (11.3%), abdominal pain (7.6%), and headache (5.7%). Risk factors for AEs included age, overweight status, concomitant medication use, chronic illness, and fasting before MDA.
Conclusions: Triple therapy with IDA is safe and well-tolerated, with some mild-to-moderate and transient adverse events among LF-infected individuals. The high incidence of AEs highlights the need for safety monitoring during MDA. The significant reductions in LF antigenemia prevalence and high CFA clearance rates underscore IDA's effectiveness in reducing LF transmission, positioning it as a key strategy for eliminating LF as a public health problem by 2030.
Keywords: Active safety surveillance; Adverse events; Albendazole; Diethylcarbamazine; Efficacy; Ivermectin; Lymphatic filariasis; Mass drug administration; Positive participants.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study received ethical approval from the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (Ref No: KNH-ERC/A/413). Written informed consent and/or assent were obtained from adults, parents, or legal guardians of children before study enrolment. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Triple-drug therapy with ivermectin, diethylcarbamazine and albendazole for the acceleration of lymphatic filariasis elimination in Kenya: Programmatic implementation and results of the first impact assessment.PLoS Negl Trop Dis. 2024 Jul 8;18(7):e0011942. doi: 10.1371/journal.pntd.0011942. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 38976718 Free PMC article.
-
Efficacy of ivermectin and albendazole combination in suppressing transmission of lymphatic filariasis following mass administration in Tanzania: a prospective cohort study.Infect Dis Poverty. 2024 Jun 12;13(1):44. doi: 10.1186/s40249-024-01214-3. Infect Dis Poverty. 2024. PMID: 38867265 Free PMC article.
-
An open label, block randomized, community study of the safety and efficacy of co-administered ivermectin, diethylcarbamazine plus albendazole vs. diethylcarbamazine plus albendazole for lymphatic filariasis in India.PLoS Negl Trop Dis. 2021 Feb 16;15(2):e0009069. doi: 10.1371/journal.pntd.0009069. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33591979 Free PMC article. Clinical Trial.
-
Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature.PLoS Negl Trop Dis. 2018 May 16;12(5):e0006454. doi: 10.1371/journal.pntd.0006454. eCollection 2018 May. PLoS Negl Trop Dis. 2018. PMID: 29768412 Free PMC article. Review.
-
Antifilarial treatment strategies: a systematic review and network meta-analysis.BMC Infect Dis. 2025 May 16;25(1):712. doi: 10.1186/s12879-025-11105-z. BMC Infect Dis. 2025. PMID: 40380307 Free PMC article. Review.
References
-
- Lymphatic filariasis. https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis. Accessed 15 Oct 2024.
-
- Moraga P, Cano J, Baggaley RF, Gyapong JO, Njenga SM, Nikolay B, et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub-Saharan Africa prior to scaling up interventions: integrated use of geostatistical and mathematical modelling. Parasit Vectors. 2015;8:560. - PMC - PubMed
-
- Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. https://www.who.int/publications/i/item/9241547103. Accessed 5 Sep 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

