Neuronal guidance signaling in neurodegenerative diseases: Key regulators that function at neuron-glia and neuroimmune interfaces
- PMID: 39995079
- PMCID: PMC12220729
- DOI: 10.4103/NRR.NRR-D-24-01330
Neuronal guidance signaling in neurodegenerative diseases: Key regulators that function at neuron-glia and neuroimmune interfaces
Abstract
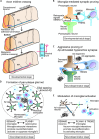
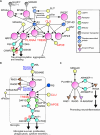
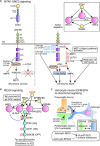
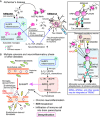
The nervous system processes a vast amount of information, performing computations that underlie perception, cognition, and behavior. During development, neuronal guidance genes, which encode extracellular cues, their receptors, and downstream signal transducers, organize neural wiring to generate the complex architecture of the nervous system. It is now evident that many of these neuroguidance cues and their receptors are active during development and are also expressed in the adult nervous system. This suggests that neuronal guidance pathways are critical not only for neural wiring but also for ongoing function and maintenance of the mature nervous system. Supporting this view, these pathways continue to regulate synaptic connectivity, plasticity, and remodeling, and overall brain homeostasis throughout adulthood. Genetic and transcriptomic analyses have further revealed many neuronal guidance genes to be associated with a wide range of neurodegenerative and neuropsychiatric disorders. Although the precise mechanisms by which aberrant neuronal guidance signaling drives the pathogenesis of these diseases remain to be clarified, emerging evidence points to several common themes, including dysfunction in neurons, microglia, astrocytes, and endothelial cells, along with dysregulation of neuron-microglia-astrocyte, neuroimmune, and neurovascular interactions. In this review, we explore recent advances in understanding the molecular and cellular mechanisms by which aberrant neuronal guidance signaling contributes to disease pathogenesis through altered cell-cell interactions. For instance, recent studies have unveiled two distinct semaphorin-plexin signaling pathways that affect microglial activation and neuroinflammation. We discuss the challenges ahead, along with the therapeutic potentials of targeting neuronal guidance pathways for treating neurodegenerative diseases. Particular focus is placed on how neuronal guidance mechanisms control neuron-glia and neuroimmune interactions and modulate microglial function under physiological and pathological conditions. Specifically, we examine the crosstalk between neuronal guidance signaling and TREM2, a master regulator of microglial function, in the context of pathogenic protein aggregates. It is well-established that age is a major risk factor for neurodegeneration. Future research should address how aging and neuronal guidance signaling interact to influence an individual's susceptibility to various late-onset neurological diseases and how the progression of these diseases could be therapeutically blocked by targeting neuronal guidance pathways.
Keywords: TDP-43; TREM2; amyloid-β; axon guidance; neurodegeneration; neuroimmune interactions; neuroinflammation; neuron-glia interactions; neurovascular interactions; semaphorin; synaptic remodeling; tau; α-synuclein.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures

References
-
- Albanus RD, et al. Systematic analysis of cellular crosstalk reveals a role for SEMA6D-TREM2 regulating microglial function in Alzheimer’s disease. BioRxiv [preprint] 2023 doi: 10.1101/2022.11.11.516215.
-
- Alberti S, Hyman AA. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol. 2021;22:196–213. - PubMed
LinkOut - more resources
Full Text Sources

