Synthesis, structure, ionochromic and cytotoxic properties of new 2-(indolin-2-yl)-1,3-tropolones
- PMID: 39996166
- PMCID: PMC11849549
- DOI: 10.3762/bjoc.21.26
Synthesis, structure, ionochromic and cytotoxic properties of new 2-(indolin-2-yl)-1,3-tropolones
Abstract
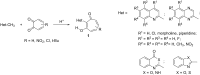
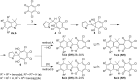
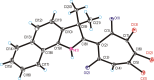
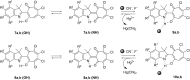
The acid-catalyzed reaction of benzo[e(g)] derivatives of 2,3,3-trimethylindolenines with o-chloranil leads to new 2-(benzo[e(g)]indolin-2-yl)-5,6,7-trichloro-1,3-tropolones and 2-(benzo[e(g)]indolin-2-yl)-4,5,6,7-tetrachloro-1,3-tropolones. Based on the results of PBE0/6-311+G(d,p) calculations, the structural and energetic characteristics of the tautomeric forms of the obtained 1,3-tropolones were determined. The structure of 2-(3,3-dimethyl-3H-benzo[g]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone was determined by X-ray diffraction analysis. The compounds obtained are capable of switching emission at 420-440 nm and 476-530 nm upon successive exposure to CN- and Hg2+ ions in an acetonitrile solution. 2-(1,1-Dimethyl-1H-benzo[e]indolin-2-yl)-5,6,7-trichloro-1,3-tropolone exhibited high in vitro cytotoxic activity against A431 skin cancer and H1299 lung cancer cell lines.
Keywords: 1,3-tropolones; X-ray diffraction; cytotoxic activity; fluorescence; o-chloranil; quantum chemical DFT calculations.
Copyright © 2025, Sayapin et al.
Figures





Similar articles
-
2-Hetaryl-1,3-tropolones based on five-membered nitrogen heterocycles: synthesis, structure and properties.Beilstein J Org Chem. 2015 Nov 12;11:2179-88. doi: 10.3762/bjoc.11.236. eCollection 2015. Beilstein J Org Chem. 2015. PMID: 26664640 Free PMC article.
-
Reaction of quinaldine with 4,6-di(tert-butyl)-3-nitro-1,2-benzoquinone. Dependence of the outcome on the reaction conditions and a deeper insight into the mechanism.Heliyon. 2023 Jun 7;9(6):e16943. doi: 10.1016/j.heliyon.2023.e16943. eCollection 2023 Jun. Heliyon. 2023. PMID: 37389047 Free PMC article.
-
Synthesis, Characterization, Quantum-Chemical Calculations and Cytotoxic Activity of 1,8-Naphthalimide Derivatives with Non-Protein Amino Acids.Anticancer Agents Med Chem. 2019;19(10):1276-1284. doi: 10.2174/1871520619666190307115231. Anticancer Agents Med Chem. 2019. PMID: 30848212
-
Relationship between electronic structure and cytotoxic activity of tropolones.In Vivo. 2006 May-Jun;20(3):391-5. In Vivo. 2006. PMID: 16724677
-
Synthesis of N-substituted 3-(2-aryl-2-oxoethyl)-3-hydroxyindolin-2-ones and their conversion to N-substituted (E)-3-(2-aryl-2-oxoethylidene)indolin-2-ones: synthetic sequence, spectroscopic characterization and structures of four 3-hydroxy compounds and five oxoethylidene products.Acta Crystallogr C Struct Chem. 2020 May 1;76(Pt 5):433-445. doi: 10.1107/S2053229620004143. Epub 2020 Apr 20. Acta Crystallogr C Struct Chem. 2020. PMID: 32367824 Free PMC article.
References
-
- Minkin V I, Aldoshin S M, Komissarov V N, Dorogan I V, Sayapin Y A, Tkachev V V, Starikov A G. Russ Chem Bull. 2006;55:2032–2055. doi: 10.1007/s11172-006-0547-x. - DOI
-
- Sayapin Y A, Komissarov V N, Bang D N, Dorogan I V, Minkin V I, Tkachev V V, Shilov G V, Aldoshin S M, Charushin V N. Mendeleev Commun. 2008;18(4):180–182. doi: 10.1016/j.mencom.2008.07.002. - DOI
-
- Sayapin Y A, Gusakov E A, Kolodina A A, Komissarov V N, Dorogan I V, Tkachev V V, Shilov G V, Nosova E V, Aldoshin S M, Charushin V N, et al. Russ Chem Bull. 2014;63:1364–1372. doi: 10.1007/s11172-014-0604-9. - DOI
-
- Sayapin Y A, Gusakov E A, Dorogan I V, Tupaeva I O, Teimurazov M G, Fursova N K, Ovchinnikov K V, Minkin V I. Russ J Bioorg Chem. 2016;42:224–228. doi: 10.1134/s1068162016020114. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
