Indole derivatives ameliorated the methamphetamine-induced depression and anxiety via aryl hydrocarbon receptor along "microbiota-brain" axis
- PMID: 39996473
- PMCID: PMC11864316
- DOI: 10.1080/19490976.2025.2470386
Indole derivatives ameliorated the methamphetamine-induced depression and anxiety via aryl hydrocarbon receptor along "microbiota-brain" axis
Abstract
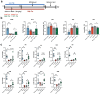
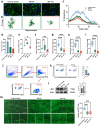
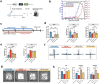
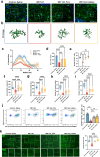
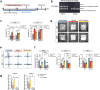
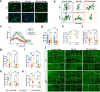
In addition to the high neurotoxicity, depression, and anxiety are the most prominent characteristics of methamphetamine (Meth) withdrawal. Studies to date on the issue of Meth-associated depression and anxiety are focused on the brain, however, whether peripheral homeostasis, especially the "microbiota-gut" axis participates in these adverse outcomes, remains poorly understood. In the current study, with the fecal microbiota transplantation (FMT) assay, the mice received microbiota from Meth withdrawal mice displayed marked depression and anxiety behaviors. The 16S rRNA sequencing results showed that Meth withdrawal contributed to a striking reduction of Akkermansia, Bacteroides, Faecalibaculum, Desulfovibrio, and Anaerostipes, which are known to be associated with tryptophan (TRP) metabolism. Noteworthily, the substantial decreases of the indole derivatives from the TRP metabolic pathway, including IAA, IPA, ILA, IET, IArA, IAld, and TRM were observed in the serum of both Meth abusing humans and mice during Meth withdrawal with the UHPLC-MS/MS analysis. Combining the high and low TRP diet mouse model, the mice with high TRP diet obviously impeded Meth-associated depression and anxiety behaviors, and these results were further strengthened by the evidence that administration of IPA, IAA, and indole dramatically ameliorated the Meth induced aberrant behaviors. Importantly, these protective effects were remarkably counteracted in aryl hydrocarbon receptor knockout (AhR KO) mice, underlining the key roles of microbiota-indoles-AhR signaling in Meth-associated depression and anxiety. Collectively, the important contribution of the present work is that we provide the first evidence that peripheral gut homeostasis disturbance but not limited to the brain, plays a key role in driving the Meth-induced depression and anxiety in the periods of withdrawal, especially the microbiota and the indole metabolic disturbance. Therefore, targeting AhR may provide novel insight into the therapeutic strategies for Meth-associated psychological disorders.
Keywords: Depression and anxiety; aryl hydrocarbon receptor; indole derivatives; methamphetamine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- World Drug Report 2023 . UNODC; 2023.
-
- Ma J, Sun X-J, Wang R-J, Wang T-Y, Su M-F, Liu M-X, Li S-X, Han Y, Meng S-Q, Wu P, et al. Profile of psychiatric symptoms in methamphetamine users in China: greater risk of psychiatric symptoms with a longer duration of use. Psychiat Res. 2018;262:184–192. doi:10.1016/j.psychres.2018.02.017. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
