Cranial ultrasound in neonatal brain infections
- PMID: 39996578
- PMCID: PMC12237230
- DOI: 10.1111/dmcn.16279
Cranial ultrasound in neonatal brain infections
Abstract
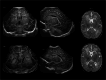
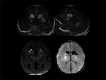
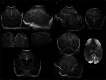
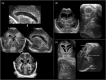
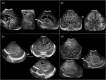
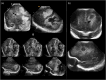
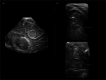
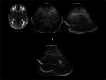
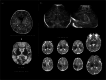
Infection of the neonatal central nervous system (CNS) can cause irreversible brain damage. Cranial ultrasound is an important neuroimaging modality in the neonatal period for detecting brain injury. Several types of organism can cause neonatal CNS infection. The aim of this narrative review is to provide an overview of the most common and typical ultrasonographic features of neonatal CNS infections and their evolution over time. Different microorganisms cause characteristic brain injury patterns. Using numerous imaging examples, we explain the different injury patterns caused by several Gram-positive and Gram-negative microorganisms, fungi, and viruses. This can guide the clinician to appropriate diagnosis and treatment.
© 2025 The Author(s). Developmental Medicine & Child Neurology published by John Wiley & Sons Ltd on behalf of Mac Keith Press.
Figures




References
-
- Hallmaier‐Wacker LK, Andrews A, Hope R, Demirjian A, Lamagni TL, Collin SM. Incidence of infant Gram‐negative invasive bacterial infections in England, 2011‐2019: an observational study using population‐wide surveillance data. Arch Dis Child. 2023. Sep;108(9):762–767. - PubMed
-
- Okike IO, Johnson AP, Henderson KL, Blackburn RM, Muller‐Pebody B, Ladhani SN, et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged. Clinical infectious diseases 2014;59(10):e150–e157. - PubMed
-
- Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert AA, McDonald J, et al. The epidemiology, management, and outcomes of bacterial meningitis in infants. Pediatrics. 2017. Jul;140(1):e20170476. - PubMed
-
- Polin RA, Harris MC. Neonatal bacterial meningitis. Semin Neonatol 2001;6: 157–172. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

