Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth
- PMID: 39996721
- PMCID: PMC11852824
- DOI: 10.3390/cells14040248
Combinational Inhibition of MEK and AKT Synergistically Induces Melanoma Stem Cell Apoptosis and Blocks NRAS Tumor Growth
Abstract
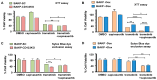
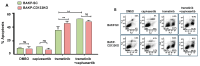
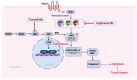
Malignant melanoma is a lethal skin cancer containing melanoma-initiating cells (MICs), implicated in tumorigenesis, invasion, and drug resistance, and characterized by an elevated expression of stem cell markers, including CD133. siRNA knockdown of CD133 has been previously shown to enhance apoptosis induced by the MEK inhibitor trametinib in melanoma cells. This study investigates the underlying mechanisms of CD133's anti-apoptotic activity in patient-derived BAKP melanoma, harboring the difficult-to-treat NRASQ61K driver mutation, after CRISPR-Cas9 CD133 knockout or Doxycycline (Dox)-inducible re-expression of CD133. CD133 knockout in BAKP cells increased trametinib-induced apoptosis by reducing anti-apoptotic p-AKT and p-BAD and increasing pro-apoptotic BAX. Conversely, Dox-induced CD133 expression diminished apoptosis in trametinib-treated cells, coincident with elevated p-AKT, p-BAD, and decreased activation of BAX and caspase-3. However, trametinib in combination with pan-AKT inhibitor capivasertib reduced cell survival as measured by XTT viability assays and apoptosis and colony formation assays, independent of CD133 status. CD133 may therefore activate a survival pathway wherein (1) increased AKT phosphorylation and activation induces (2) BAD phosphorylation and inactivation, which (3) decreases BAX activation, and (4) reduces caspases-3 activity and caspase-mediated PARP cleavage, leading to apoptosis suppression and drug resistance in melanoma. In vivo mouse xenograft studies using Dox-inducible melanoma cells revealed increased rates of tumor growth after induction of CD133 expression in trametinib-treated +Dox mice, an effect which was synergistically suppressed by combination treatment. Targeting nodes of the AKT and MAPK survival pathways with trametinib and capivasertib highlights the potential for combination therapies for NRAS-mutant melanoma stem cells for the development of more effective treatments for patients with high-risk melanoma.
Keywords: CRISPR-Cas9; NRAS; capivasertib; drug resistance; trametinib; xenograft.
Conflict of interest statement
Author Peter Sykora was employed by the company Amelia Technologies, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- SEER . Cancer Statistics-Reports on Cancer-Cancer Stat Facts-Melanoma of the Skin. Surveillance, Epidemiology, and End Results (SEER) Program; Bethesda, MD, USA: 2024. [(accessed on 1 December 2024)]. Available online: www.seer.cancer.gov.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

