Radiation Retinopathy: Microangiopathy-Inflammation-Neurodegeneration
- PMID: 39996770
- PMCID: PMC11853965
- DOI: 10.3390/cells14040298
Radiation Retinopathy: Microangiopathy-Inflammation-Neurodegeneration
Abstract
Purpose: Proton irradiation is used to treat choroidal melanoma of the eye. The impact on non-malignant retinal cells is currently understudied. Therefore, we here report a mouse model to investigate the impact of proton irradiation on the retina.
Methods: We performed a proton beam irradiation of 5-15 Cobalt-Gray-Equivalent (CGE) of the eyes of female C57Bl6/J (Cx3cr1+/+), Cx3cr1gfp/+ and Cx3cr1gfp/gfp mice mimicking the clinical situation and evaluated the structure, function and cellular composition of the retina up to 24 weeks after irradiation.
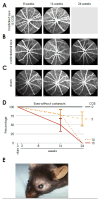
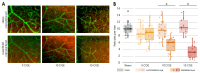
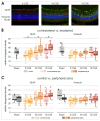
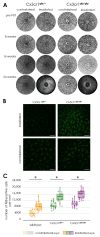
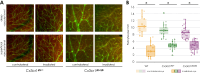
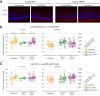
Results: Proton beam irradiation of the eye with 15 CGE leads to cataract formation after 24 weeks without affecting the gross anatomy of the retinal vasculature as shown by Fundus imaging in all genotypes respectively. However, 10 and 15 CGE, lead to a significant decrease in NG2 positive cell numbers and all three dosages induced an increase in GFAP immunoreactivity. At 24 weeks a dosage of 15 CGE resulted in functional impairment and a decrease of NG2 positive cells in both WT and Cx3cr1 animals. Iba1 cell immunoreactivity was increased in all genotypes. However, in the Cx3cr1 animals the invasion of Iba1 cells into the deep vascular layer was partially prevented. This was accompanied by a less severe functional impairment in the irradiated Cx3cr1gfp/gfp vs. WT.
Conclusions: Although the gross anatomy of the retina does not seem to be affected by proton beam irradiation, the cellular composition and retinal function changed significantly in both WT and Cx3cr1 mice reflecting the clinical situation. Moreover, cataract formation was one of the major long-term effects of irradiation. We conclude that the murine model (WT and Cx3cr1 genotype) can be used to investigate proton-beam associated side effects in vivo as well as to test prospective interventions. Moreover, the loss of Cx3cr1 seems to be partially protective.
Keywords: radiation; retina; retinopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Desjardins L., Levy C., d’Hermies F., Frau E., Schlienger P., Habrand J.L., Mammar H., Schwartz L., Mazal A., Delacroix S., et al. Initial results of proton therapy in choroidal melanoma at the d’Orsey Center for Proton Therapy; the first 464 cases. Cancer Radiother. 1997;1:222–226. doi: 10.1016/S1278-3218(97)89768-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

