A Critical Role of Intracellular PD-L1 in Promoting Ovarian Cancer Progression
- PMID: 39996786
- PMCID: PMC11853747
- DOI: 10.3390/cells14040314
A Critical Role of Intracellular PD-L1 in Promoting Ovarian Cancer Progression
Abstract
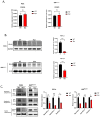
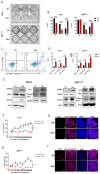
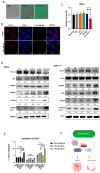
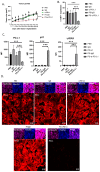
Disrupting the interaction between tumor-cell surface PD-L1 and T cell membrane PD-1 can elicit durable clinical responses. However, only about 10% of ovarian cancer patients respond to PD-1/PD-L1 blockade. Here, we show that PD-L1 expression in ovarian cancer-patient tumors is predominantly intracellular. Notably, PARP inhibitor treatment highly increased intracellular PD-L1 accumulation in both ovarian cancer-patient tumor samples and cell lines. We investigated whether intracellular PD-L1 might play a critical role in ovarian cancer progression. Mutating the PD-L1 acetylation site in PEO1 and ID8Brca1-/- ovarian cancer cells significantly decreased PD-L1 levels and impaired colony formation, which was accompanied by cell cycle G2/M arrest and apoptosis induction. PEO1 and ID8Brca1-/- tumors with PD-L1 acetylation site mutation also exhibited significantly reduced growth in mice. Furthermore, targeting intracellular PD-L1 with a cell-penetrating antibody effectively decreased ovarian tumor-cell intracellular PD-L1 level and induced tumor-cell growth arrest and apoptosis, as well as enhanced DNA damage and STING activation, both in vitro and in vivo. In conclusion, we have shown the critical role of intracellular PD-L1 in ovarian cancer progression.
Keywords: intracellular PD-L1; ovarian cancer; progression.
Conflict of interest statement
The cell-penetrating antibody technology patent has been licensed to Inova through City of Hope. H.Y. is a co-inventor on the patent. The other authors have no conflicts of interest declared.
Figures

References
-
- Hamanishi J., Mandai M., Ikeda T., Minami M., Kawaguchi A., Murayama T., Kanai M., Mori Y., Matsumoto S., Chikuma S., et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. - DOI - PubMed
-
- Varga A., Piha-Paul S., Ott P.A., Mehnert J.M., Berton-Rigaud D., Morosky A., Yang P., Ruman J., Matei D. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019;152:243–250. doi: 10.1016/j.ygyno.2018.11.017. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

