Respiratory Muscle Injury Following Acute Monocled Cobra (Naja kaouthia) Envenoming: Histopathological Study in Rat Diaphragm
- PMID: 39996807
- PMCID: PMC11854468
- DOI: 10.3390/cimb47020086
Respiratory Muscle Injury Following Acute Monocled Cobra (Naja kaouthia) Envenoming: Histopathological Study in Rat Diaphragm
Abstract
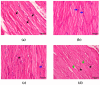
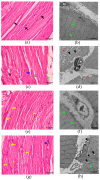
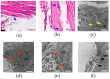
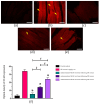
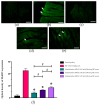
Clinical symptoms of monocled cobra (Naja kaouthia) envenoming include the paralysis of extraocular muscles, local tissue necrosis and death through respiratory failure. These neurotoxic outcomes are mainly due to the inhibitory action of postsynaptic neurotoxins to nicotinic acetylcholine receptors. However, injuries involving respiratory muscles have rarely been investigated. In this study, we determined the effect of N. kaouthia envenoming on morphological changes in the rat diaphragm. The efficacy of cobra monovalent antivenom in neutralising the histopathological effects of N. kaouthia venom was also evaluated. The intramuscular (i.m.) administration of N. kaouthia venom (2 mg/kg) caused skeletal muscle fibre atrophy and ruptures of myofibrils shown via a light microscope study. Transmission electron microscopy (TEM) revealed the zig-zagging of the Z-band, mitochondrial damages and degeneration of the synaptic fold of the neuromuscular junction following experimental cobra envenoming for 4 h. Intravenous administration of cobra antivenom at manufacturer-recommended doses diminished histopathological changes in the diaphragm following the administration of cobra venom. The expression of NF-kB and MuRF1 in the experimentally N. kaouthia-envenomed diaphragm indicated inflammation and tissue atrophy in the immunofluorescence analysis, respectively. In this study, we found that there were respiratory muscle injuries following N. kaouthia envenoming. The early administration of monovalent N. kaouthia antivenom is capable of neutralising neurotoxic outcomes following cobra envenoming.
Keywords: antivenom; diaphragm; histology; myotoxicity; snakebite.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Geographical venom variations of the Southeast Asian monocled cobra (Naja kaouthia): venom-induced neuromuscular depression and antivenom neutralization.Comp Biochem Physiol C Toxicol Pharmacol. 2016 Jul-Aug;185-186:77-86. doi: 10.1016/j.cbpc.2016.03.005. Epub 2016 Mar 10. Comp Biochem Physiol C Toxicol Pharmacol. 2016. PMID: 26972756
-
Biochemical and biological characterization of the venoms of Naja kaouthia and Naja mandalayensis from Myanmar and neutralization effects of BPI cobra antivenom.Toxicon X. 2024 Apr 3;22:100196. doi: 10.1016/j.toxcx.2024.100196. eCollection 2024 Jun. Toxicon X. 2024. PMID: 38665175 Free PMC article.
-
Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia.J Proteomics. 2015 Apr 29;120:105-25. doi: 10.1016/j.jprot.2015.02.012. Epub 2015 Mar 5. J Proteomics. 2015. PMID: 25748141
-
Antivenom for Neuromuscular Paralysis Resulting From Snake Envenoming.Toxins (Basel). 2017 Apr 19;9(4):143. doi: 10.3390/toxins9040143. Toxins (Basel). 2017. PMID: 28422078 Free PMC article. Review.
-
Composition, pharmacology, and pathophysiology of the venom of monocled cobra (Naja kaouthia)- a medically crucial venomous snake of southeast Asia: An updated review.Toxicon. 2024 Oct;249:108056. doi: 10.1016/j.toxicon.2024.108056. Epub 2024 Aug 5. Toxicon. 2024. PMID: 39111718 Review.
References
-
- WHO . Guidelines for the Management of Snakebites. 2nd ed. WHO; Geneva, Switzerland: 2016. [(accessed on 10 September 2023)]. Available online: https://www.who.int/publications/i/item/9789290225300.
-
- WHO . Guidelines for the Management of Snake-Bites. WHO; Geneva, Switzerland: 2010. [(accessed on 5 December 2022)]. Available online: https://iris.who.int/bitstream/handle/10665/204464/B4508.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources

