Design and Development of Natural-Product-Derived Nanoassemblies and Their Interactions with Alpha Synuclein
- PMID: 39997105
- PMCID: PMC11852371
- DOI: 10.3390/biomimetics10020082
Design and Development of Natural-Product-Derived Nanoassemblies and Their Interactions with Alpha Synuclein
Abstract
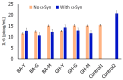
Biomimetic nanoassemblies derived from natural products are considered promising nanomaterials due to their self-assembling ability and their favorable interactions with biological molecules leading to their numerous applications as therapeutic agents or as molecular probes. In this work, we have created peptide nanoconjugates of two natural products, β-Boswellic acid (BA) and β-glycyrrhetinic acid (GH). Both BA and GH are known for their medicinal value, including their role as strong antioxidants, anti-inflammatory, neuroprotective and as anti-tumor agents. To enhance the bioavailability of these molecules, they were functionalized with three short peptides (YYIVS, MPDAHL and GSGGL) to create six conjugates with amphiphilic structures capable of facile self-assembly. The peptides were also derived from natural sources and have been known to display antioxidant activity. Depending upon the conjugate, nanofibers, nanovesicles or a mixture of both were formed upon self-assembly. The binding interactions of the nanoconjugates with α-Synuclein, a protein implicated in Parkinson's disease (PD) was examined through in silico studies and FTIR, circular dichroism and imaging studies. Our results indicated that the nanoassemblies interacted with alpha-synuclein fibrils efficaciously. Furthermore, the nanoassemblies were found to demonstrate high viability in the presence of microglial cells, and were found to enhance the uptake and interactions of α-Synuclein with microglial cells. The nanoconjugates designed in this work may be potentially utilized as vectors for peptide-based drug delivery or for other therapeutic applications.
Keywords: alpha synuclein; cellular uptake; nanoassemblies; self-assembly; terpenes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
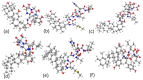

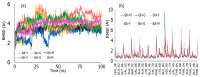
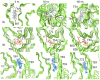
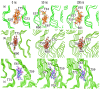
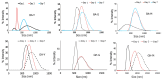


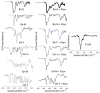
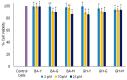

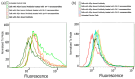
Similar articles
-
Peptide-Based Nanoassemblies in Gene Therapy and Diagnosis: Paving the Way for Clinical Application.Molecules. 2020 Jul 31;25(15):3482. doi: 10.3390/molecules25153482. Molecules. 2020. PMID: 32751865 Free PMC article. Review.
-
Exploring the Potential of Biomimetic Peptides in Targeting Fibrillar and Filamentous Alpha-Synuclein-An In Silico and Experimental Approach to Parkinson's Disease.Biomimetics (Basel). 2024 Nov 18;9(11):705. doi: 10.3390/biomimetics9110705. Biomimetics (Basel). 2024. PMID: 39590277 Free PMC article.
-
Interactions of Nanoscale Self-Assembled Peptide-Based Assemblies with Glioblastoma Cell Models and Spheroids.ACS Omega. 2023 Mar 22;8(13):12124-12143. doi: 10.1021/acsomega.2c08049. eCollection 2023 Apr 4. ACS Omega. 2023. PMID: 37033803 Free PMC article.
-
Biomimetic nanoassemblies of 1-O-octodecyl-2-conjugated linoleoyl-sn-glycero-3-phosphatidyl gemcitabine with phospholipase A2-triggered degradation for the treatment of cancer.Colloids Surf B Biointerfaces. 2017 Apr 1;152:467-474. doi: 10.1016/j.colsurfb.2017.02.001. Epub 2017 Feb 3. Colloids Surf B Biointerfaces. 2017. PMID: 28187380
-
Anti-amyloid potential of some phytochemicals against Aβ-peptide and α-synuclein, tau, prion, and Huntingtin protein.Drug Discov Today. 2023 Dec;28(12):103802. doi: 10.1016/j.drudis.2023.103802. Epub 2023 Oct 17. Drug Discov Today. 2023. PMID: 37858630 Review.
References
-
- Tang N., Ding Z., Zhang S., Luo D., Liu X., Bao X., Liu C., Liu Z. Nanoassemblies derived from natural flavonoid compounds as new antioxidant oral preparations for targeted inflammatory bowel disease therapy. Adv. Funct. Mater. 2023;33:2305133. doi: 10.1002/adfm.202305133. - DOI
-
- Serrano Vega R.J., Campos Xolalpa N., Castro Alonso J.A., González Pérez C., Pérez Ramos J., Pérez Gutiérrez S. Terpenes from Natural Products with Potential Anti- Activity Inflammatory Activity. In: Perveen S., Al-Taweel A., editors. Terpenes from Natural Products with Potential Anti-Inflammatory Activity. IntechOpen; London, UK: 2018. pp. 59–85. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

