Candidemia in ICU Patients: What Are the Real Game-Changers for Survival?
- PMID: 39997446
- PMCID: PMC11855959
- DOI: 10.3390/jof11020152
Candidemia in ICU Patients: What Are the Real Game-Changers for Survival?
Abstract
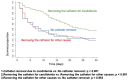
Candidemia infection remains a critical challenge in intensive care units (ICUs), with high morbidity and mortality rates despite advances in therapeutic practices. This multicenter prospective surveillance study assessed the epidemiology, clinical management, and mortality predictors of candidemia in critically ill patients across two periods (2010-2012 and 2017-2018) in 11 tertiary hospitals in Brazil. Among 314 ICU patients with candidemia, the overall mortality rate was 60.2%, with no significant reduction over time (58.8% vs. 62.6%, p = 0.721). Candida albicans was the predominant pathogen (43.6%), followed by C. tropicalis (20%) and C. glabrata (13.7%). The use of echinocandins increased significantly in the second period (21.1% to 41.7%, p < 0.001); however, 70% of patients still did not receive these agents as first-line therapy. Catheter removal due to candidemia was performed in only 52.1% of cases but was associated with improved 30-day survival (p < 0.001). Multivariate analysis identified cancer, inadequate treatment, and vasoactive drug use as independent predictors of mortality. Our findings underscore persistent gaps in adherence to guidelines, particularly regarding timely echinocandin initiation and catheter removal. Strengthening therapeutic strategies focused on these key interventions is essential to improving outcomes for ICU patients with candidemia.
Keywords: cancer; candidemia; catheter removal; echinocandins; intensive care unit.
Conflict of interest statement
C.A. has received support for attending educational meetings from Merck Sharp & Dohme (MSD), Pfizer, and United Medical. A.L.C. has received educational grants from Sandoz, Knight-United Medical, Mundipharma, Pfizer, Gilead Sciences, and United Medical. T.G. has received educational grants from Merck Sharp & Dohme (MSD). F.Q.-T. has received consulting fees for Pfizer, TEVA, and United Medical. T.S. has received support for attending meetings from Pfizer and Merck Sharp & Dohme (MSD). M.M.C.M has received support for attending educational meetings from Knight and Mundipharma. The remaining authors declare no conflict of interest.
Figures
References
-
- World Health Organization WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. [(accessed on 28 November 2024)]. Available online: https://www.who.int/publications/i/item/9789240060241.
-
- Buetti N., Tabah A., Loiodice A., Ruckly S., Aslan A.T., Montrucchio G., Cortegiani A., Saltoglu N., Kayaaslan B., Aksoy F., et al. Different epidemiology of bloodstream infections in COVID-19 compared to non-COVID-19 critically ill patients: A descriptive analysis of the Eurobact II study. Crit. Care. 2022;26:319. doi: 10.1186/s13054-022-04166-y. - DOI - PMC - PubMed
-
- Nucci M., Queiroz-Telles F., Alvarado-Matute T., Tiraboschi I.N., Cortes J., Zurita J., Guzman-Blanco M., Santolaya M.E., Thompson L., Sifuentes-Osornio J., et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE. 2013;8:e59373. doi: 10.1371/journal.pone.0059373. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources