The Potential Role of Adipose-Derived Stem Cells in Regeneration of Peripheral Nerves
- PMID: 39997654
- PMCID: PMC11858299
- DOI: 10.3390/neurolint17020023
The Potential Role of Adipose-Derived Stem Cells in Regeneration of Peripheral Nerves
Abstract
Peripheral nerve injuries are common complications in surgical and dental practices, often resulting in functional deficiencies and reduced quality of life. Current treatment choices, such as autografts, have limitations, including donor site morbidity and suboptimal outcomes. Adipose-derived stem cells (ADSCs) have shown assuring regenerative potential due to their accessibility, ease of harvesting and propagation, and multipotent properties. This review investigates the therapeutic potential of ADSCs in peripheral nerve regeneration, focusing on their use in bioengineered nerve conduits and supportive microenvironments. The analysis is constructed on published case reports, organized reviews, and clinical trials from Phase I to Phase III that investigate ADSCs in managing nerve injuries, emphasizing both peripheral and orofacial applications. The findings highlight the advantages of ADSCs in promoting nerve regeneration, including their secretion of angiogenic and neurotrophic factors, support for cellular persistence, and supplementing scaffold-based tissue repair. The regenerative capabilities of ADSCs in peripheral nerve injuries offer a novel approach to augmenting nerve repair and functional recovery. The accessibility of adipose tissue and the minimally invasive nature of ADSC harvesting further encourage its prospective application as an autologous cell source in regenerative medicine. Future research is needed to ascertain standardized protocols and optimize clinical outcomes, paving the way for ADSCs to become a mainstay in nerve regeneration.
Keywords: adipose tissue-derived stem cells; adipose-derived stromal cells; nerve conduits; peripheral nerve injury; peripheral nerve repair and regeneration; regenerative medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

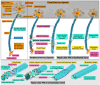

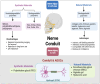
Similar articles
-
Augmenting Peripheral Nerve Regeneration with Adipose-Derived Stem Cells.Stem Cell Rev Rep. 2022 Feb;18(2):544-558. doi: 10.1007/s12015-021-10236-5. Epub 2021 Aug 20. Stem Cell Rev Rep. 2022. PMID: 34417730 Free PMC article. Review.
-
Adipose derived stem cells for the peripheral nerve regeneration: review of techniques and clinical implications.J Pak Med Assoc. 2023 Feb;73(Suppl 1)(2):S148-S154. doi: 10.47391/JPMA.AKUS-24. J Pak Med Assoc. 2023. PMID: 36788407 Review.
-
The Effect of Adipose-Derived Mesenchymal Stem Cells on Peripheral Nerve Damage in a Rodent Model.J Clin Med. 2023 Oct 9;12(19):6411. doi: 10.3390/jcm12196411. J Clin Med. 2023. PMID: 37835055 Free PMC article.
-
Effect of Breast Cancer and Adjuvant Therapy on Adipose-Derived Stromal Cells: Implications for the Role of ADSCs in Regenerative Strategies for Breast Reconstruction.Stem Cell Rev Rep. 2021 Apr;17(2):523-538. doi: 10.1007/s12015-020-10038-1. Epub 2020 Sep 14. Stem Cell Rev Rep. 2021. PMID: 32929604 Review.
-
Adipose-derived stem cells modified by TWIST1 silencing accelerates rat sciatic nerve repair and functional recovery.Hum Cell. 2024 Sep;37(5):1394-1404. doi: 10.1007/s13577-024-01087-6. Epub 2024 Jun 21. Hum Cell. 2024. PMID: 38907140 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

