Is N 1-Methylnicotinamide a Good Organic Cation Transporter 2 (OCT2) Biomarker?
- PMID: 39997705
- PMCID: PMC11857448
- DOI: 10.3390/metabo15020080
Is N 1-Methylnicotinamide a Good Organic Cation Transporter 2 (OCT2) Biomarker?
Abstract
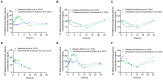
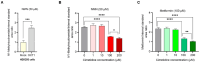
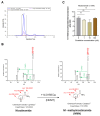
Background/Objectives: The impact of potential precipitant drugs on plasma or urinary exposure of endogenous biomarkers is emerging as an alternative approach to evaluating drug-drug interaction (DDI) liability. N1-Methylnicotinamide (NMN) has been proposed as a potential biomarker for renal organic cation transporter 2 (OCT2). NMN is synthesized in the liver from nicotinamide by nicotinamide N-methyltransferase (NNMT) and is subsequently metabolized by aldehyde oxidase (AO). Multiple clinical studies have shown a reduction in NMN plasma concentration following the administration of OCT inhibitors such as cimetidine, trimethoprim, and pyrimethamine, which contrasts with their inhibition of NMN renal clearance by OCT2. We hypothesized that OCT1-mediated NMN release from hepatocytes is inhibited by the administration of OCT inhibitors. Methods: Re-analysis of the reported NMN pharmacokinetics with and without OCT inhibitor exposure was performed. We assessed the effect of cimetidine on NMN uptake in OCT1-HEK293 cells and evaluated the potential confounding effects of cimetidine on enzymes involved in NMN formation and metabolism. Results: A re-analysis of previous NMN pharmacokinetic DDI data suggests that NMN plasma systemic exposure decreased by 17-41% during the first 4 h following different OCT inhibitor administration except dolutegravir. Our findings indicate that NMN uptake was significantly higher (by 2.5-fold) in OCT1-HEK293 cells compared to mock cells, suggesting that NMN is a substrate of OCT1. Additionally, our results revealed that cimetidine does not inhibit NNMT and AO activity. Conclusions: Our findings emphasize the limitations of using NMN as an OCT2 biomarker and reveal potential mechanisms behind the reduction in NMN plasma levels associated with OCT inhibitors. Instead, our data suggest that NMN could be tested further as a potential biomarker for OCT1 activity.
Keywords: drug–drug interactions; endogenous biomarkers; hepatic transport; organic cation transport; pharmacokinetic; renal transport.
Conflict of interest statement
Bhagwat Prasad is a co-founder of Precision Quantomics Inc. and a recipient of research funding from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, Generation Bio, Gilead, Merck, Novartis, and Takeda. Zsuzsanna Gaborik is an employee of Charles River Laboratories Hungary Kft, which develops and commercializes reagents and assays to study membrane transporters. The paper reflects the views of the scientists, and not the company. All other authors declared no conflicts of interests in this work.
Figures


References
-
- Galetin A., Brouwer K.L.R., Tweedie D., Yoshida K., Sjöstedt N., Aleksunes L., Chu X., Evers R., Hafey M.J., Lai Y., et al. Membrane Transporters in Drug Development and as Determinants of Precision Medicine. Nat. Rev. Drug Discov. 2024;23:255–280. doi: 10.1038/s41573-023-00877-1. - DOI - PMC - PubMed
-
- Zamek-Gliszczynski M.J., Chu X., Cook J.A., Custodio J.M., Galetin A., Giacomini K.M., Lee C.A., Paine M.F., Ray A.S., Ware J.A., et al. ITC Commentary on Metformin Clinical Drug–Drug Interaction Study Design That Enables an Efficacy- and Safety-Based Dose Adjustment Decision. Clin. Pharmacol. Ther. 2018;104:781–784. doi: 10.1002/cpt.1082. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

