"Time to Change": To What Extent Could Non-Sputum Sampling Accelerate the Fight Against Tuberculosis-A Qualitative Study Among End-Users
- PMID: 39998048
- PMCID: PMC11860771
- DOI: 10.3390/tropicalmed10020044
"Time to Change": To What Extent Could Non-Sputum Sampling Accelerate the Fight Against Tuberculosis-A Qualitative Study Among End-Users
Abstract
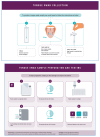
Inadequate access to timely diagnosis and linkage to treatment are major barriers to tuberculosis (TB) care. New point-of-care diagnostics that do not rely solely on sputum samples are needed to make up for lost time, bringing TB testing closer to service recipients and addressing current sputum sampling limitations. Urine-based TB lipoarabinomannan tests and tongue dorsum swabs have demonstrated potential as alternatives to sputum-based molecular testing. We conducted a study to ascertain the perceived value of these non-sputum-based TB tests among stakeholders from the TB community, including TB service recipients and healthcare providers, in India, South Africa, and Viet Nam. Our results showed that there was a high degree of enthusiasm among various end-users for both novel sample types. It is important to generate both qualitative and quantitative evidence to support optimal uptake and implementation of these potential new sample types for TB testing.
Keywords: diagnosis; end-users; non-sputum sampling; qualitative study; tongue swabs; tuberculosis; urine.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- World Health Organization . Global Tuberculosis Report 2021. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 11 March 2024)]. Available online: https://www.who.int/publications-detail-redirect/9789240037021.
Grants and funding
LinkOut - more resources
Full Text Sources