α-Latrotoxin Actions in the Absence of Extracellular Ca2+ Require Release of Stored Ca2
- PMID: 39998090
- PMCID: PMC11860464
- DOI: 10.3390/toxins17020073
α-Latrotoxin Actions in the Absence of Extracellular Ca2+ Require Release of Stored Ca2
Abstract
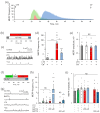
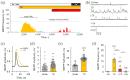
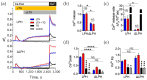
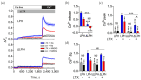
α-Latrotoxin (αLTX) causes exhaustive release of neurotransmitters from nerve terminals in the absence of extracellular Ca2+ (Ca2+e). To investigate the mechanisms underlying this effect, we loaded mouse neuromuscular junctions with BAPTA-AM. This membrane-permeable Ca2+-chelator demonstrates that Ca2+e-independent effects of αLTX require an increase in cytosolic Ca2+ (Ca2+cyt). We also show that thapsigargin, which depletes Ca2+ stores, induces neurotransmitter release, but inhibits the effect of αLTX. We then studied αLTX's effects on Ca2+cyt using neuroblastoma cells expressing signaling-capable or signaling-incapable variants of latrophilin-1, a G protein-coupled receptor of αLTX. Our results demonstrate that αLTX acts as a cation ionophore and a latrophilin agonist. In model cells at 0 Ca2+e, αLTX forms membrane pores and allows the influx of Na+; this reverses the Na+-Ca2+ exchanger, leading to the release of stored Ca2+ and inhibition of its extrusion. Concurrently, αLTX stimulates latrophilin signaling, which depletes a Ca2+ store and induces transient opening of Ca2+ channels in the plasmalemma that are sensitive to inhibitors of store-operated Ca2+ entry. These results indicate that Ca2+ release from intracellular stores and that Ca2+ influx through latrophilin-activated store-operated Ca2+ channels contributes to αLTX actions and may be involved in physiological control of neurotransmitter release at nerve terminals.
Keywords: ADGRL1; calcium; intracellular Ca2+ stores; latrophilin-1; neuroblastoma cells; neuromuscular junction; neurotransmitter release; store-operated Ca2+ entry; α-Latrotoxin.
Conflict of interest statement
Author Evelina Petitto was employed by Ashfield MedComms, The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

