Thermal Stability and Matrix Binding of Citrinin in the Thermal Processing of Starch-Rich Foods
- PMID: 39998103
- PMCID: PMC11860567
- DOI: 10.3390/toxins17020086
Thermal Stability and Matrix Binding of Citrinin in the Thermal Processing of Starch-Rich Foods
Abstract
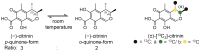
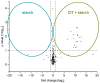
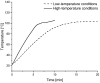
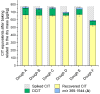
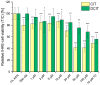
Citrinin (CIT) is a nephrotoxic mycotoxin commonly found in a broad range of foods, including cereals, spices, nuts, or Monascus fermentation products. Analyses have shown that CIT is present in processed foods in significantly lower concentrations than in unprocessed materials. Modified forms of CIT arising during food processing may provide an explanation for the discrepancy. This study deals with the thermal stability of CIT and the formation of reaction products of CIT with carbohydrates, followed by toxicological evaluations using cell culture models. HPLC-HRMS degradation curves of CIT heated in different matrix model systems were recorded, and the formation of decarboxycitrinin (DCIT), the main degradation product, was quantified. Additionally, chemical structures of reaction products of CIT with carbohydrates were tentatively identified using MS/MS spectra and stable isotope labelling. Subsequently, the degradation of CIT during biscuit baking was studied, and carbohydrate-bound forms of CIT were detected after enzymatic starch digestion. The formation of DCIT could explain the majority of CIT degradation, but, depending on the process, covalent binding to carbohydrates can also be highly relevant. Cytotoxicity of DCIT in IHKE-cells was found to be lower compared to CIT, while the toxicity as well as the intestinal metabolism of carbohydrate-bound CIT was not evaluated.
Keywords: citrinin; degradation; food; matrix binding; modified mycotoxin; mycotoxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

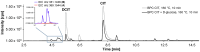

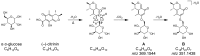
References
-
- EFSA Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012;10:2605. doi: 10.2903/j.efsa.2012.2605. - DOI
-
- López Sánchez P., de Nijs M., Spanjer M., Pietri A., Bertuzzi T., Starski A., Postupolski J., Castellari M., Hortós M. Generation of occurrence data on citrinin in food. EFSA J. 2017;14:1177E. doi: 10.2903/sp.efsa.2017.EN-1177. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

