Functional Mitral Valve Regurgitation, Pathophysiology, Leaflet ReModeling, and the Role of Imaging
- PMID: 39998385
- PMCID: PMC11854047
- DOI: 10.1111/echo.70101
Functional Mitral Valve Regurgitation, Pathophysiology, Leaflet ReModeling, and the Role of Imaging
Abstract
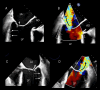
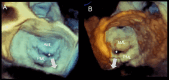
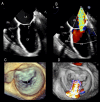
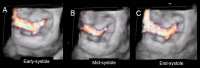
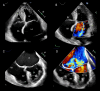
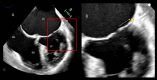
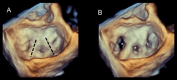
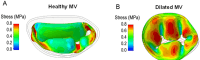
Functional mitral regurgitation (FMR) is a complex left ventricle (LV) and left atrium (LA) disorder in which mitral valve regurgitation is just the "tip of the iceberg." Unlike primary mitral cvalve regurgitation, in which regurgitation occurs due to anatomic abnormalities of the valve itself, the etiology of FMR is multifactorial. Regional and global LV dysfunction, extent and location of fibrotic myocardium (subendocardial/transmural scar), and annulus enlargement are the leading causes of valve regurgitation. A comprehensive understanding of the causes, mechanisms, severity, and clinical consequences of FMVR relies primarily on noninvasive imaging techniques. Echocardiography is the first-line and most commonly used imaging technique. Cardiac magnetic resonance (CMR) has gained growing consensus mainly because it can precisely identify the extent and location of fibrotic myocardium. This review aims to: (a) describe the pathophysiology of the most common phenotypes of FMR, (b) challenge the paradigm that mitral leaflets are structurally normal in FMR, and (c) illustrate the critical role of both echocardiography and CMR in the comprehensive assessment of FMR.
Keywords: functional mitral regurgitation; mitral valve; valve disease.
© 2025 The Author(s). Echocardiography published by Wiley Periodicals LLC.
Conflict of interest statement
Francesco F Faletra has received speker fee from Philips.
Figures

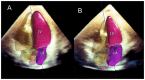


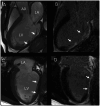
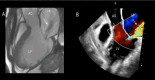
References
-
- Asgar A. W., Mack M. J., and Stone G. W., “Secondary Mitral Regurgitation in Heart Failure: Pathophysiology, Prognosis, and Therapeutic Considerations,” Journal of the American College of Cardiology 65 (2015): 1231–1248. - PubMed
-
- Agricola E., Oppizzi M., Pisani M., et al., “Ischemic Mitral Regurgitation: Mechanisms and Echocardiographic Classification,” European Journal of Echocardiography 9, no. 2 (2008): 207–221. - PubMed
-
- Landi A., Faletra F. F., Giulia Pavon A., et al., “From Secondary to Tertiary Mitral Regurgitation: The Paradigm Shifts, but Uncertainties Remain,” European Heart Journal—Cardiovascular Imaging 22, no. 8 (2021): 835–843. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

