Prognostic value of folate-associated gene expression in stage II colon cancer
- PMID: 39998667
- PMCID: PMC11861115
- DOI: 10.1007/s00432-025-06141-w
Prognostic value of folate-associated gene expression in stage II colon cancer
Abstract
Purpose: Prognostic variability in stage II colon cancer underscores the need for better risk stratification. Analyzing folate-associated gene expression in stage II colon cancer could provide researchers and clinicians with deeper insights into tumor biology and potentially aid in identifying early prognostic and/or predictive biomarkers.
Methods: Patients with stage II colon cancer and recurrence (n = 48) were matched to patients with a 5 year recurrence-free follow-up (n = 133). Gene expression of ABCC3, AMT, FPGS, GGH, MFT, PCFT, RFC-1, and TYMS was analyzed in tumor tissue and matching colon mucosa using qPCR and evaluated in relation to time to recurrence (TTR), as well as to demographic and clinicopathological variables.
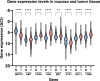
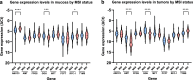
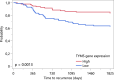
Results: Independent of other covariates, TYMS expression in tumors, pT4 stage, and emergency surgery were associated with TTR. There were significant differences in expression levels of all examined genes between tumor and mucosa. ABCC3, GGH, and RFC-1 expression levels differed in mucosa between microsatellite instability-high (MSI-H) compared to microsatellite stable/microsatellite instability-low (MSS/MSI-L) tumors, whereas tumoral expression of AMT, GGH, and TYMS differed between MSI-H and MSS/MSI-L tumors. Depending on tumor location, the expression of ABCC3, AMT, GGH, and RFC-1 in mucosa, as well as the tumoral expression of AMT, GGH, PCFT and RFC-1 differed.
Conclusion: Low tumoral expression of TYMS was associated with worse TTR, independent of MSI status, pT stage, and emergency surgery. The indication of a better outcome for patients with MSI-H status and high tumoral TYMS expression might be of particular interest in the stratification of patients for immunotherapy.
Keywords: Biomarker; Cancer; Colorectal Neoplams; Folic acid; Prognosis; Thymidylate synthase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflicts of interest. Ethical approval: The study was conducted in line with the Declaration of Helsinki, and the regional ethical review board in Gothenburg approved the study (ethical board number 590-15). Consent to participate: Written informed consent to participate was obtained from all participants.
Figures


References
-
- Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D (2020) Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up<sup>†</sup>. Ann Oncol 31(10):1291–1305. 10.1016/j.annonc.2020.06.022 - PubMed
-
- Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM (2007) Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86(1):159–166. 10.1093/ajcn/86.1.159 - PubMed
-
- Duthie SJ (2011) Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis [journal article]. J Inherit Metab Dis 34(1):101–109. 10.1007/s10545-010-9128-0 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

