Generation of frameshift-mutated TGFβR2-specific T cells in healthy subjects following administration with cancer vaccine candidate FMPV-1/GM-CSF in a phase 1 study
- PMID: 39998682
- PMCID: PMC11861775
- DOI: 10.1007/s00262-025-03969-6
Generation of frameshift-mutated TGFβR2-specific T cells in healthy subjects following administration with cancer vaccine candidate FMPV-1/GM-CSF in a phase 1 study
Abstract
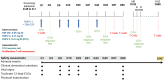
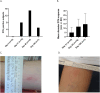
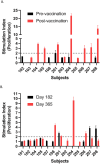
FMPV-1 is a component of FMPV-3, an investigational cancer-specific vaccine and being developed to activate anti-cancer T cell responses targeting frameshift mutations of MSI-H cancers. FMPV-1 is designed to activate T cell responses against transforming growth factor β receptor 2 (TGFβR2) frameshift mutation. Microsatellite instability high (MSI-H) gastrointestinal cancers frequently harbour TGFβR2 frameshift mutations. This first-in-human, phase 1, single centre, open-label study included 16 healthy male subjects who received FMPV-1 (0.15 mg/injection) plus granulocyte-macrophage colony-stimulating factor (GM-CSF) (0.03 mg/injection) as two separate, co-located, injections on Days 1, 8, 15, 29 and 43. All subjects were followed to Day 365. A FMPV-1-specific delayed type hypersensitivity (DTH) skin reactivity test was performed with FMPV-1 (without GM-CSF) on Days 1, 29 and 43 with assessment after 2 days. All subjects were DTH negative at baseline, 8/16 were positive on Day 31 and 15/16 were positive on Day 45. Furthermore, the FMPV-1/GM-CSF induced frameshift mutant TGFβR2-specific T cells after the short vaccination period, and specific T cells were still detectable after 6 and 12 months indicating induction of frameshift mutant TGFβR2-specific T memory cells. Adverse events were limited to mild injection site reactions with no evidence of related systemic signs or symptoms. No other clinically important changes to vital signs, electrocardiograms, haematological, coagulation or laboratory measures related to treatment were observed. FMPV-1/GM-CSF was well tolerated and generated vaccine-specific T cell immune responses in healthy subjects. These findings support clinical studies in patients with, or at risk of, cancers carrying TGFβR2 frameshift mutations.Clinical trial identification: ClinicalTrials.gov: NCT05238558. EudraCT: 2020-004363-80.
Keywords: GM-CSF; Immune response; Immunotherapy; Lynch syndrome; Peptide vaccine; TGFβR2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: NS is an employee of Quotient Sciences which was contracted by Hubro Therapeutics AS to conduct the study. RMM, SA-B, HKE, BI, JAE and KRH were employed by study sponsor. EMI and HVJ are employees of Translational Research Unit, Section for Cellular Therapy, Department of Oncology, Oslo University Hospital—Radiumhospitalet, Oslo, Norway. The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. Ethics approval and consent to participate: This study was approved by the UK Medicines and Healthcare products Regulatory Agency and the protocol approved by the London – West London & Gene Therapy Advisory Committee Research Ethics Committee, the Norwegian National Research Ethics Committee and written informed consent was obtained from all subjects. Consent for publication: The authors declare that they consent for publication.
Figures
References
-
- Kloor M, von Knebel DM (2016) The immune biology of microsatellite-unstable cancer. Trends Cancer 2(3):121–133. 10.1016/j.trecan.2016.02.004 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

