The MEK5/ERK5 pathway promotes the activation of the Hedgehog/GLI signaling in melanoma cells
- PMID: 39998753
- PMCID: PMC12119679
- DOI: 10.1007/s13402-025-01050-z
The MEK5/ERK5 pathway promotes the activation of the Hedgehog/GLI signaling in melanoma cells
Abstract
Purpose: Malignant melanoma is the deadliest skin cancer, with a poor prognosis in advanced stages. We reported that both Hedgehog-GLI (HH/GLI) and Mitogen-activated protein Kinase (MAPK) extracellular signal-regulated kinase 5 (ERK5) pathways promote melanoma growth, and that ERK5 activation is required for HH/GLI-dependent melanoma cell proliferation. Here, we explored whether ERK5 regulates HH/GLI signaling.
Methods: Both genetic (using ERK5-specific shRNA) and pharmacologic (using the ERK5 inhibitors JWG-071 and AX15836, and the MAPK/ERK kinase 5, MEK5 inhibitors GW284543 and BIX02189) targeting approaches were used. Luciferase assay using the GLI-binding site luciferase reporter was performed to evaluate GLI transcriptional activity. A constitutively active form of MEK5 (MEK5DD) was used to induce ERK5 activation. 3D spheroid assays were performed in melanoma cells.
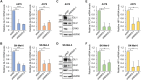
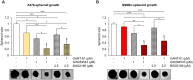
Results: Genetic and pharmacologic ERK5 inhibition reduces GLI1 and GLI2 protein levels and transcriptional activity of endogenous HH/GLI pathway induced by the agonist SAG in NIH/3T3 cells. In these cells, MEK5DD overexpression potentiates transcriptional activity of endogenous HH/GLI pathway induced by SAG, whereas ERK5 silencing prevents this effect. Consistently, MEK5DD overexpression increases GLI1 and GLI2 protein levels. In melanoma cells, ERK5 silencing reduces GLI1 and GLI2 mRNA and protein levels and inhibits GLI transcriptional activity. MEK5DD further increases the transcriptional activity of the HH/GLI pathway and GLI1 protein levels. Combination of GLI and MEK5 inhibitors is more effective than single treatments in reducing melanoma spheroid growth.
Conclusions: MEK5-ERK5 is an activator of GLI transcription factors, and combined targeting of these pathways warrants further preclinical investigation as a potential innovative therapeutic approach for melanoma.
Keywords: ERK5/MAPK7; GLI; Hedgehog; Melanoma; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not Applicable. Consent for publication: This publication has full consent from all authors. Competing interests: The authors declare no competing interests.
Figures




References
-
- Z. Lombardi, L. Gardini, A.V. Kashchuk, A. Menconi, M. Lulli, I. Tusa, A. Tubita, L. Maresca, B. Stecca, M. Capitanio, E. Rovida, Importin subunit beta-1 mediates ERK5 nuclear translocation, and its Inhibition synergizes with ERK5 kinase inhibitors in reducing cancer cell proliferation. Mol. Oncol. 4 (2024) - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

