Late Adverse Events After Chimeric Antigen Receptor T-Cell Therapy for Patients With Aggressive B-Cell Non-Hodgkin Lymphoma
- PMID: 39998830
- PMCID: PMC11862965
- DOI: 10.1001/jamanetworkopen.2024.61683
Late Adverse Events After Chimeric Antigen Receptor T-Cell Therapy for Patients With Aggressive B-Cell Non-Hodgkin Lymphoma
Abstract
Importance: Acute adverse events (AEs) after chimeric antigen receptor (CAR) T-cell infusion are well documented, but less information is available regarding the long-term toxic effects.
Objective: To assess the occurrence of late AEs for adult patients with large B-cell lymphoma (LBCL) treated with commercially available CD19-targeted CAR T cells.
Design, setting, and participants: A prospective, observational, clinical practice cohort study was conducted from September 1, 2018, to December 31, 2022, among 172 adult patients in 6 Spanish hospitals who received CD19-targeted CAR T-cell therapy for relapsed or refractory LBCL and survived at least 3 months after infusion, without subsequent antilymphoma therapy.
Exposure: Treatment with tisagenlecleucel or axicabtagene ciloleucel.
Main outcomes and measures: Data on any late AEs occurring in this patient population were collected until the patients received new antilymphoma therapy, were lost to follow-up, died, or reached 24 months after infusion, whichever occurred first. Data collection for each patient started at the third month after infusion and included new-onset AEs, as well as persistent AEs that started earlier but were still ongoing at that time point.
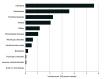
Results: The study enrolled 172 patients (mean [SD] age, 58.5 [13.7] years; 101 men [58.7%]), of whom 135 (78.5%) experienced at least 1 late AE of any grade. Infections were the late AEs with the highest incidence (5.6 per 100 person-months [95% CI, 4.5-7.0 per 100 person-months]), followed by neutropenia (3.6 per 100 person-months [95% CI, 2.9-4.5 per 100 person-months]) and thrombocytopenia (2.2 per 100 person-months [95% CI, 1.7-3.0 per 100 person-months]). The incidence of infectious episodes remained stable during the whole study period, while cytopenias decreased beyond 6 months after infusion. All cases of nonrelapse-related mortality were due to infections (COVID-19 pneumonia in 3 patients and sepsis or bacterial pneumonia in 4 patients). Twenty-three patients (13.4%) experienced 27 dermatologic AEs, all mild, with most of them (88.9% [24 of 27]) starting beyond 3 months after infusion. Fifteen neurologic AEs were reported in 15 patients (8.7%), and 10 patients (5.8%) developed 13 cardiovascular AEs. Five secondary neoplasms were reported in 4 patients (2.3%), with no cases of T-cell malignant neoplasms.
Conclusions and relevance: This cohort study suggests that CAR T-cell therapy has a favorable safety profile. However, continuous follow-up of patients is needed, as serious AEs can occur years after infusion.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources