Sustainability-Inspired Upcycling of Organophosphorus Pollutants into Phosphatic Fertilizer in a Continuous-Flow Reactor
- PMID: 39998984
- PMCID: PMC12051758
- DOI: 10.1002/anie.202502408
Sustainability-Inspired Upcycling of Organophosphorus Pollutants into Phosphatic Fertilizer in a Continuous-Flow Reactor
Abstract
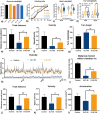
With the increasing requirement for phosphorus resources and their shortage in nature, cyclic utilization of organophosphorus pollutants into phosphatic fertilizer might offer a sustainable approach to achieve the recycling of phosphorus. Herein, we first report the selective degradation of organophosphorus pollutants, via the synergistic effect of peroxymonosulfate (PMS) and sodium percarbonate (SPC), into phosphates (o-PO4 3-), which are continually converted into phosphatic fertilizer by struvite precipitation on the continuous-flow reactor. Quenching experiments, electron paramagnetic resonance (EPR) results, electrochemical analysis, and density functional theory (DFT) calculation suggest that the transfer of electrons from SPC to PMS results in the synthesis of catalytically active species (i.e., ·OH, ·O2 -, 1O2, and CO3·-) for hydroxyethylidene-1,1-diphosphonicacid (HEDP) degradation. For the real glyphosate wastewater, the PMS/SPC system exhibits excellent catalytic activity with 69.20% decrease in chemical oxygen demand (COD) and 37.80% decrease in the total organic carbon (TOC) after 90 min. Indeed, high performance liquid chromatography (HPLC) confirms that glyphosate is completely degraded in 90 min with the formation of 271.93 µmol/L of o-PO4 3-, which is further converted into phosphatic fertilizer by the precipitation of struvite with 87.20% yield on continuous-flow reactor. Finally, biotoxicity of glyphosate to zebrafish and wheat seeds are significantly deceased after treatment of PMS/SPC system by zebrafish toxicology assays and germination tests of wheat seeds.
Keywords: Biotoxicity; Continuous‐flow reaction; Organophosphorus pollutants; Phosphatic fertilizer; Struvite.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Degradation of Organophosphorus Pollutants into Inorganic Phosphates by Co(II)/NaBO3 System and Its Phosphorus Recycling.Inorg Chem. 2025 Jul 7;64(26):13396-13404. doi: 10.1021/acs.inorgchem.5c01797. Epub 2025 Jun 19. Inorg Chem. 2025. PMID: 40534486
-
Sustainability-inspired upcycling of plastic waste into porous carbon nanobulks for water decontamination via peroxymonosulfate activation.Sci Total Environ. 2024 Nov 10;950:175242. doi: 10.1016/j.scitotenv.2024.175242. Epub 2024 Aug 8. Sci Total Environ. 2024. PMID: 39117214
-
MOFs-derived porous carbon embedded Fe0 nanoparticles as peroxymonosulfate activator for efficient degradation of organic pollutants.Environ Res. 2025 Mar 1;268:120790. doi: 10.1016/j.envres.2025.120790. Epub 2025 Jan 8. Environ Res. 2025. PMID: 39793875
-
Complete and rapid degradation of glyphosate with Fe3Ce1Ox catalyst for peroxymonosulfate activation at room temperature.Environ Res. 2021 Oct;201:111618. doi: 10.1016/j.envres.2021.111618. Epub 2021 Jul 6. Environ Res. 2021. PMID: 34237337
-
MOFs-derived magnetic C@Cu-Ni bimetal particles: An efficient peroxymonosulfate activator for 2,4,6-trichlorophenol degradation.Chemosphere. 2021 Apr;269:129394. doi: 10.1016/j.chemosphere.2020.129394. Epub 2020 Dec 21. Chemosphere. 2021. PMID: 33388568
References
-
- Weidenmüller A., Meltzer A., Neupert S., Kleineidam C., Science 2022, 376, 1122–1126. - PubMed
-
- Peng W., Lam S. S., Sonne C., Science 2020, 367, 257–258. - PubMed
-
- Hofstetter T. B., Bakkour R., Buchner D., Eisenmann H., Fischer A., Gehre M., Haderlein S. B., Höhener P., Hunkeler D., Imfeld G., Jochmann M. A., Kümmel S., Martin P. R., Pati S. G., Schmidt T. C., Vogt C., Elsner M., Nat. Water 2024, 2, 14–30.
-
- Huang N., Xu Z., Wang W., Wang Q., Wu Q., Hu H., Sep. Purif. Technol. 2022, 297, 121385.
Grants and funding
LinkOut - more resources
Full Text Sources

