The Association Between Metabolic Dysfunction-Associated Steatotic Liver Disease and Change in Liver Stiffness in Patients With Chronic Hepatitis B
- PMID: 39999021
- PMCID: PMC11855902
- DOI: 10.1111/liv.70042
The Association Between Metabolic Dysfunction-Associated Steatotic Liver Disease and Change in Liver Stiffness in Patients With Chronic Hepatitis B
Abstract
Background and aims: Metabolic dysfunction-associated steatotic liver disease (MASLD) is associated with an increased risk of liver-related events in patients with chronic hepatitis B (CHB), possibly by accelerating fibrosis progression. Therefore, we studied the influence of MASLD on liver stiffness measurement (LSM) kinetics in CHB patients.
Methods: We conducted a multicenter retrospective cohort study of CHB patients with at least two LSM with FibroScan. We studied the absolute change in LSM and the change in LSM stage from the first LSM to the most recent LSM among CHB patients with MASLD compared to patients without MASLD.
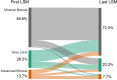
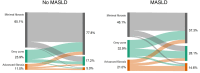
Results: We analysed 1055 CHB patients; 259 (28.0%) had MASLD. Patients with MASLD had a higher first and last LSM (6.1 vs. 5.2 kPa and 5.6 vs. 4.7 kPa, p < 0.001), were significantly less likely to achieve a decrease in LSM stage (52.8% vs. 74% p < 0.001) and were more likely to experience an increase in LSM stage (19.3% vs. 13.6%, p = 0.035) during follow-up. 417 (39.5%) patients initiated antiviral therapy (AVT) which was associated with a decline in LSM (p < 0.001). However, patients with MASLD who were treated were less likely to decrease in LSM stage (52.4% vs. 77.0%, p < 0.001) and were more likely to experience an increase in LSM stage (23.5% vs. 12.8%, p = 0.021) despite AVT.
Conclusion: Presence of MASLD was independently associated with higher LSM in untreated CHB patients and with less decline in LSM after initiation of AVT. Furthermore, CHB patients with MASLD were more likely to experience an increase in LSM despite AVT.
Keywords: MAFLD; MASLD; NAFLD; liver stiffness measurement; metabolic comorbidities.
Liver International© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
L.A.P. received a research grant from Gilead Sciences. R.J.d.K. received a grant from Echosens, Gilead Sciences, GlaxoSmithKline, Inventiva, Janssen‐Cialg. Consultancy and/or speakers fee from Abbvie, Gilead Sciences, Janssen‐Cilag, Schallware. H.L.A.J. received grants from: Gilead Sciences, GlaxoSmithKline, Janssen, Roche, Vir Biotechnology. Consultant for: Aligos, Gilead Sciences, GlaxoSmithKline, Grifols, Roche, Vir Biotechnology Inc., Precision Biosciences. W.P.B. received a speakers fee from Eli Lilly. Is on the advisory board of Novo Nordisk. Participated in trials from Inventive pharma, Boehringer Ingelheim and 89BIO. M.K. received a speakers fee from Norgine. J.d.B. research support from Terumo. D.F.P. Member of DSMB of the COBRA‐trial (Very short‐course versus standard course antibiotic therapy in patients with acute ChOlangitis after adequate endoscopic BiliaRy drAinage [COBRA trial]; consultancy fees from Gilead [payed to institution]). R.A.d.M. research support from Roche. M.J.S. Speakers fees and research support from Gilead, Roche, Fujirebio and consultancy fees from Gilead and Albireo. K.v.E., Ö.M.K., P.H., G.J.B., H.B., and R.B.T. none.
Figures
References
-
- World Health Organization , “Fact Sheet—Hepatitis B 2024,” updated 09‐04‐2024, https://www.who.int/news‐room/fact‐sheets/detail/hepatitis‐b.
-
- Papatheodoridis G. V., Chan H. L., Hansen B. E., Janssen H. L., and Lampertico P., “Risk of Hepatocellular Carcinoma in Chronic Hepatitis B: Assessment and Modification With Current Antiviral Therapy,” Journal of Hepatology 62, no. 4 (2015): 956–967. - PubMed
-
- Marcellin P., Gane E., Buti M., et al., “Regression of Cirrhosis During Treatment With Tenofovir Disoproxil Fumarate for Chronic Hepatitis B: A 5‐Year Open‐Label Follow‐Up Study,” Lancet 381, no. 9865 (2013): 468–475. - PubMed
-
- Arends P., Sonneveld M. J., Zoutendijk R., et al., “Entecavir Treatment Does Not Eliminate the Risk of Hepatocellular Carcinoma in Chronic Hepatitis B: Limited Role for Risk Scores in Caucasians,” Gut 64, no. 8 (2015): 1289–1295. - PubMed
-
- Eslam M., Newsome P. N., Sarin S. K., et al., “A New Definition for Metabolic Dysfunction‐Associated Fatty Liver Disease: An International Expert Consensus Statement,” Journal of Hepatology 73, no. 1 (2020): 202–209. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical