Retrospective Analysis: S100 as Marker for Immune Effector Cell-Associated Neurotoxicity Syndrome
- PMID: 39999826
- PMCID: PMC12490844
- DOI: 10.1159/000543949
Retrospective Analysis: S100 as Marker for Immune Effector Cell-Associated Neurotoxicity Syndrome
Abstract
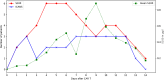
Introduction: Chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising treatment for hematologic malignancies, offering significant therapeutic benefits. However, this therapy is also associated with adverse effects such as cytokine release syndrome and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS), which can lead to severe neurological symptoms. The pathophysiology of ICANS remains unclear but is believed to involve immune-mediated inflammation in the brain. This study investigates the potential of S100, a protein marker associated with blood-brain barrier integrity, as an early indicator of ICANS.
Methods: We retrospectively analyzed daily blood samples for S100 levels in patients undergoing CAR T-cell therapy, correlating these levels with the onset and severity of ICANS.
Results: The results show that S100 levels significantly increased in patients who developed ICANS, with a positive correlation between the duration of elevation and the severity of the neurological symptoms.
Conclusion: These findings suggest that S100 may serve as a useful biomarker for early detection of ICANS and could potentially guide therapeutic interventions. However, further studies are needed to fully understand its prognostic value in this context.
Keywords: Chimeric antigen receptor T-cell therapy; ICANS; Marker; S100; Side effects.
© 2025 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
-
- San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389(4):335–47. - PubMed
-
- June CH, et al. CAR T cell therapy for patients with hematologic malignancies: a patient-centered approach. J Clin Oncol. 2018;36(12):1220–9.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources