mHealth intervention (mTB-Tobacco) for smoking cessation in people with drug-sensitive pulmonary tuberculosis in Bangladesh and Pakistan: protocol for an adaptive design, cluster randomised controlled trial (Quit4TB)
- PMID: 40000077
- PMCID: PMC12083406
- DOI: 10.1136/bmjopen-2024-089007
mHealth intervention (mTB-Tobacco) for smoking cessation in people with drug-sensitive pulmonary tuberculosis in Bangladesh and Pakistan: protocol for an adaptive design, cluster randomised controlled trial (Quit4TB)
Abstract
Introduction: People with tuberculosis (TB) who continue to smoke are more likely to have poor health outcomes than those who quit. Established smoking cessation approaches such as mHealth may help patients with TB quit smoking. This paper summarises the methodology proposed to assess the effectiveness and cost-effectiveness of mTB-Tobacco (an mHealth intervention) in helping patients with TB stop smoking and have improved health outcomes.
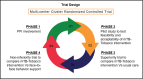
Methods and analysis: A two-arm, parallel, open-label, multicentre, cluster randomised, two-stage adaptive design trial is proposed to first evaluate the superiority of mTB-Tobacco, compared with usual care and then the non-inferiority of mTB-tobacco compared with face-to-face behaviour support. Study settings include TB treatment centres in Bangladesh and Pakistan. The study population includes adult patients, newly diagnosed (within 4 weeks) with pulmonary TB disease, daily smokers, willing to quit and have access to mobile phones. The primary outcome includes biochemically verified continuous smoking abstinence assessed at 6 months per Russell Standard. A generalised linear mixed-effects model will be used to assess the impact of mTB-Tobacco intervention on continuous outcomes, incorporating fixed effects for the intervention, random effects for clusters and relevant covariates. Cost-effectiveness analysis will be done to estimate the cost per quitter and cost per quality-adjusted life year gained, calculate the incremental cost-effectiveness ratios to establish the value for money for mTB-Tobacco.
Ethics and dissemination: This trial will be conducted in compliance with International Council on Harmonisation - Good Clinical Practice guidelines and the Declaration of Helsinki. The study has been approved by the ethics committees of the University of Edinburgh Medical School Research Ethics Committee (EMREC) of UK, the Bangladesh Medical Research Council (BMRC) and the National Bioethics Committee (PMRC) of Pakistan. The results of this trial will be disseminated in peer-reviewed journals and presented in academic conferences.
Trial registration number: ISRCTN86971818 (https://doi.org/10.1186/ISRCTN86971818); pre-enrolment, submission date: 29 August 2023; registration date: 11 September 2023.
Keywords: Behaviour; Digital Technology; Smoking Reduction; Tobacco Use; Tuberculosis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- World Health Organization . Geneva, Switzerland: World Health Organization; 2023. Global tuberculosis report 2023.https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf... Available.
-
- Marks GB, Teo AK, Wong EB, et al. The Challenge of Tuberculosis in the 21st Century: ERS Monograph 101; 2023. International efforts to reverse and end the tuberculosis pandemic: past, present and future global strategies. - DOI
-
- World Health Organization . Geneva, Switzerland: World Health Organization; 2023. https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf... Available.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
