Tebipenem pivoxil as an alternative to ceftriaxone for clinically non-responding children with shigellosis: a randomised non-inferiority trial protocol
- PMID: 40000088
- PMCID: PMC12083402
- DOI: 10.1136/bmjopen-2024-088449
Tebipenem pivoxil as an alternative to ceftriaxone for clinically non-responding children with shigellosis: a randomised non-inferiority trial protocol
Abstract
Introduction: Shigellosis is the second leading cause of diarrhoeal deaths among children worldwide. Oral azithromycin and intravenous ceftriaxone are the recommended first-line and second-line therapies for shigellosis in Bangladesh, respectively, but growing antibiotic resistance will require new antibiotic options. Tebipenem pivoxil, an orally administered carbapenem antibiotic with activity against many strains of antibiotic-resistant bacteria, may be a viable option.
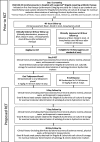
Methods: A phase IIb randomised controlled trial was planned to determine the efficacy and safety of oral tebipenem pivoxil, compared with intravenous ceftriaxone, for children with Shigella diarrhoea unresponsive to the first-line antibiotic therapy. We will enrol 132 children in the trial (66 in each arm). Children from Bangladesh aged 24-59 months suspected of having Shigella diarrhoea, with no clinical improvement within 48 hours of starting first-line therapy, will be randomised to a 3-day course of intravenous ceftriaxone (50 mg/kg, once a day) or a 3-day course of oral tebipenem pivoxil (4 mg/kg, three times a day). The children will be evaluated for key clinical, microbiological and safety outcomes during the subsequent 30-day period. Clinically, failure at day 3 will be defined as the presence of fever (axillary temperature ≥38°C), diarrhoea (three or more abnormally loose or watery stools in the last 24 hours), blood in stool, or abdominal pain/tenderness at day 3 of follow-up or death or hospitalisation prior to day 3. It is hypothesised that children treated with tebipenem pivoxil will have no worse clinical and microbiological failure rates compared with ceftriaxone.
Ethics and dissemination: This study protocol was approved by the institutional review board of the International Centre for Diarrhoeal Disease Research, Bangladesh, which comprises a research review committee and an ethics review committee. In addition, the use of tebipenem pivoxil in shigellosis was approved by the Directorate General of Drug Administration of Bangladesh.
Trial registration number: NCT05121974.
Keywords: CLINICAL PHARMACOLOGY; Clinical Trial; Paediatric gastroenterology; Protocols & guidelines.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AN, RG, EFA, and JA are employees of the GSK group of companies. AN and RG also own shares in the GSK group of companies. AN, RG, EFA, and JA declare no other financial and non-financial relationships and activities. The other authors declare that they have no competing interests.
Figures
References
-
- Octavia S, Lan R. Molecular medical microbiology. Elsevier; 2015. Shigella and shigellosis: genetics, epidemiology and pathogenesis; pp. 1147–68.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical