Results From a Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of ANX005, a C1q Inhibitor, in Patients With Guillain-Barré Syndrome
- PMID: 40000167
- PMCID: PMC11886941
- DOI: 10.1111/jns.70009
Results From a Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of ANX005, a C1q Inhibitor, in Patients With Guillain-Barré Syndrome
Erratum in
-
Correction to "Results From a Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Efficacy of ANX005, a C1q Inhibitor, in Patients With Guillain-Barré Syndrome".J Peripher Nerv Syst. 2025 Jun;30(2):e70024. doi: 10.1111/jns.70024. J Peripher Nerv Syst. 2025. PMID: 40375604 Free PMC article. No abstract available.
Abstract
Background and aims: Guillain-Barré syndrome (GBS) is an acute autoimmune peripheral neuropathy driven by autoantibodies and classical complement pathway activation. Despite treatments with intravenous immunoglobulin or plasma exchange, GBS remains characterized by variability in recovery and high incidence of residual disabilities. This randomized, double-blind, placebo-controlled Phase 1 trial evaluated the safety, tolerability, and pharmacokinetics of ANX005, a novel therapeutic targeting the classical complement cascade.
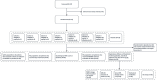
Methods: Patients with recent-onset GBS, who had no access to intravenous immunoglobulin or plasma exchange, received escalating doses of ANX005 or placebo as a single IV infusion. Primary objectives included assessments of safety. Secondary objectives included determination of pharmacokinetic and pharmacodynamic profiles and clinical outcomes through Week 8. Exploratory objectives included an evaluation of serum and cerebrospinal fluid (CSF) complement and tissue damage biomarkers.
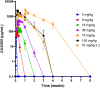
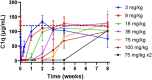
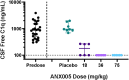
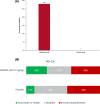
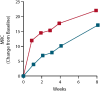
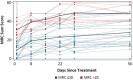
Results: Fifty patients were randomized to receive either ANX005 (n = 38) or placebo (n = 12). ANX005 was well-tolerated across all doses with no dose-limiting toxicities, suggesting an acceptable safety profile. Pharmacodynamic data showed effective C1q inhibition and a reduction in neurofilament light chain levels, suggesting nerve damage mitigation. Exploratory endpoints evaluating clinical outcomes included improvements in Medical Research Council sum scores, GBS-Disability Score, and Overall Neuropathy Limitations Scale with ANX005 compared to placebo, particularly in patients receiving doses that inhibited serum C1q for ≥ 1 week and provided C1q blockade in the CSF.
Interpretation: These results support ANX005 as a targeted therapy for GBS that modulates the classical complement pathway. Further investigation in a larger Phase 3 trial is warranted to confirm these results and assess the long-term benefits of complement inhibition in patients with GBS.
Keywords: ANX005; C1q; Guillain–Barré syndrome; Phase 1; complement.
© 2025 Peripheral Nerve Society.
Conflict of interest statement
Quazi Deen Mohammad reports a consultancy/advisory role with Annexon Biosciences. Zhahirul Islam reports research funding from Fogarty International Center, National Institute of Neurological Disorders and Stroke of the National Institutes of Health, USA, and Annexon Biosciences. Nowshin Papri, Shoma Hayat, Israt Jahan, and Khan Abul Kalam Azad report no disclosures relevant to the manuscript. Dean R. Artis and Benjamin Hoehn are employees of Annexon Biosciences. Eric Humphriss is a former employee and shareholder of Annexon Biosciences. Ted Yednock is an employee of Annexon Biosciences and holds equity ownership in Annexon Biosciences. Henk‐André Kroon is an employee and shareholder of Annexon Biosciences.
Figures

References
-
- Willison H. J., Jacobs B. C., and van Doorn P. A., “Guillain‐Barré Syndrome,” Lancet 388, no. 10045 (2016): 717–727. - PubMed
-
- van den Berg B., Walgaard C., Drenthen J., Fokke C., Jacobs B. C., and van Doorn P. A., “Guillain‐Barré Syndrome: Pathogenesis, Diagnosis, Treatment and Prognosis,” Nature Reviews. Neurology 10, no. 8 (2014): 469–482. - PubMed
-
- van Doorn P. A., “Diagnosis, Treatment and Prognosis of Guillain‐Barré Syndrome (GBS),” Presse Médicale 42, no. 6 Pt 2 (2013): e193–e201. - PubMed
-
- Fokke C., van den Berg B., Drenthen J., Walgaard C., van Doorn P. A., and Jacobs B. C., “Diagnosis of Guillain‐Barré Syndrome and Validation of Brighton Criteria,” Brain 137, no. Pt 1 (2014): 33–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

