Defining the methanogenic SECIS element in vivo by targeted mutagenesis
- PMID: 40000419
- PMCID: PMC11881835
- DOI: 10.1080/15476286.2025.2472448
Defining the methanogenic SECIS element in vivo by targeted mutagenesis
Abstract
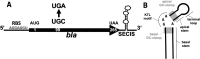
In all domains of life, Archaea, Eukarya and Bacteria, the unusual amino acid selenocysteine (Sec) is co-translationally incorporated into proteins by recoding a UGA stop codon to a sense codon. A secondary structure on the mRNA, the selenocysteine insertion sequence (SECIS), is required, but its position, secondary structure and binding partner(s) are not conserved across the tree of life. Thus far, the nature of archaeal SECIS elements has been derived mainly from sequence analyses. A recently developed in vivo reporter system was used to study the structure-function relationships of SECIS elements in Methanococcus maripaludis. Through targeted mutagenesis, we defined the minimal functional SECIS element, the parts of the SECIS where structure and not the identity of the bases are relevant for function, and identified two conserved -and invariant- adenines that are most likely to interact with the other factor(s) of the Sec recoding machinery. Finally, we demonstrated the functionality of SECIS elements in the 5`-untranslated region of the mRNA and identified a potential mechanism of SECIS repositioning in the vicinity of the UGA for efficient selenocysteine insertion.
Keywords: Archaea; Methanococcus; SECIS element; reporter; selenocysteine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes.Nat Struct Mol Biol. 2005 May;12(5):408-16. doi: 10.1038/nsmb922. Epub 2005 Apr 10. Nat Struct Mol Biol. 2005. PMID: 15821744
-
In vivo probing of SECIS-dependent selenocysteine translation in Archaea.Life Sci Alliance. 2022 Oct 31;6(1):e202201676. doi: 10.26508/lsa.202201676. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36316034 Free PMC article.
-
Characterization of the UGA-recoding and SECIS-binding activities of SECIS-binding protein 2.RNA Biol. 2014;11(11):1402-13. doi: 10.1080/15476286.2014.996472. RNA Biol. 2014. PMID: 25692238 Free PMC article.
-
Selenoprotein synthesis in archaea.Biofactors. 2001;14(1-4):75-83. doi: 10.1002/biof.5520140111. Biofactors. 2001. PMID: 11568443 Review.
-
An extended Escherichia coli "selenocysteine insertion sequence" (SECIS) as a multifunctional RNA structure.Biofactors. 2001;14(1-4):61-8. doi: 10.1002/biof.5520140109. Biofactors. 2001. PMID: 11568441 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
