Is there still a place for autologous salvage transplantation in relapsed/refractory multiple myeloma in the era of novel therapies?
- PMID: 40000504
- PMCID: PMC12031966
- DOI: 10.1007/s00277-025-06262-9
Is there still a place for autologous salvage transplantation in relapsed/refractory multiple myeloma in the era of novel therapies?
Abstract
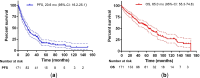
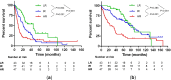
For patients (pts) with relapsed or refractory multiple myeloma (RRMM) after previous autologous hematopoietic cell transplantation (AHCT), novel agents, cellular and immunotherapies are increasingly available. Options for second-line treatment mostly include triplet regimens based on proteasome inhibitors, immunomodulatory drugs and anti-CD38 monoclonal antibodies and since recently also CAR T cells. The importance of autologous salvage transplantation (retransplantation, Re-AHCT) has significantly decreased in recent years due to the availability of many new treatment options. Therefore, we performed a retrospective analysis of 171 pts cases with RRMM who received Re-AHCT between 2002 and 2021. With a median follow-up of 74.7 months, the 5-year rates of progression-free survival (PFS) and overall survival (OS) were 18% (median 20.6 months) and 57% (median 65.0 months), respectively, the 100-day mortality rate was 4%. Multivariate analysis identified R-ISS stage and duration of previous response (DoR) as independent prognostic factors for PFS and OS. While the revealed high-risk population (R-ISS stage II/III, DoR ≤ 24 months) was associated with a significantly worse PFS (HR 2.728) and OS (HR 3.129), the low-risk group (R-ISS I, DoR > 24 months) achieved a median PFS and OS of 45.0 months and 80.2 months, respectively. Therefore, Re-AHCT could remain an option in such prognostically favorable pts with RRMM even in the era of novel therapies especially when more potent treatment modalities are not available.
Keywords: Autologous hematopoetic cell transplantation; Multiple myeloma; Relapse; Retransplantation; Salvage autologous.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study was approved by the regional ethics committees of the State Chamber of Physicians of Saxony (EK-BR-40/23 − 1). Patient consent: Patient consent was waived in accordance with the ethical approval due to retrospective design of the analysis and anonymization. Competing interests: K. Trautmann-Grill has received consultation fees and/or honoraria from Sanofi, Takeda, Novartis, Amgen, GSK, Janssen; travel grants from Janssen, NovoNordisk, GSK, Sanofi and has participated in Data Safety Monitoring Board or advisory boards for Sanofi, Novartis, Amgen, GSK. M. Hänel has received consultations fees and/or honoraria from Novartis, Amgen, GSK, Celgene, Sanofi-Aventis, Janssen, and Bristol-Myers Squibb. R. Herbst has received honoraria from Novartis, Bristol-Myers Squibb and Amgen. R. Teipel has participated in advisory boards or has received honoraria from Janssen, Bristol-Myers Squibb, Takeda, GSK, Gilead, Sanofi, Amgen, Stemline, Oncopeptides and Abbvie as well as research and travel grants from Janssen. All other authors declare no competing financial interests in relation to the work described. All authors declare that potential competing interests did not influence the content and results of the manuscript.
Figures
Similar articles
-
Effects of Consolidation Therapy With Autologous Hematopoietic Stem Cell Transplantation After BCMA-CAR T-Cell Therapy on the Survival of Patients With Relapsed or Refractory Multiple Myeloma.Transplant Cell Ther. 2024 Nov;30(11):1080.e1-1080.e11. doi: 10.1016/j.jtct.2024.08.024. Epub 2024 Sep 3. Transplant Cell Ther. 2024. PMID: 39236790
-
Is Salvage Autologous Stem Cell Transplantation Still a Viable Treatment Option in Relapsed Myeloma Patients?Medicina (Kaunas). 2025 May 7;61(5):859. doi: 10.3390/medicina61050859. Medicina (Kaunas). 2025. PMID: 40428816 Free PMC article.
-
Outcomes of Daratumumab, Pomalidomide, and Dexamethasone, Followed by High-dose Chemotherapy and Autologous Stem Cell Transplantation, in Patients With Relapsed/Refractory Multiple Myeloma.Clin Lymphoma Myeloma Leuk. 2021 Feb;21(2):e212-e219. doi: 10.1016/j.clml.2020.08.026. Epub 2020 Sep 18. Clin Lymphoma Myeloma Leuk. 2021. PMID: 33051166
-
Autologous hematopoietic stem cell transplantation for multiple myeloma in the age of CAR T cell therapy.Front Oncol. 2024 Mar 27;14:1373548. doi: 10.3389/fonc.2024.1373548. eCollection 2024. Front Oncol. 2024. PMID: 38601770 Free PMC article. Review.
-
From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives.Haematologica. 2018 Feb;103(2):197-211. doi: 10.3324/haematol.2017.174573. Epub 2017 Dec 7. Haematologica. 2018. PMID: 29217780 Free PMC article. Review.
References
-
- Garderet L, Cook G, Auner HW, Bruno B, Lokhorst H, Perez-Simon JA et al (2017) Treatment options for relapse after autograft in multiple myeloma – report from an EBMT educational meeting. Leuk Lymphoma 58(4):797–808. 10.1080/10428194.2016.1228926 - PubMed
-
- Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D et al (2020) Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet 396(10245):186–197. 10.1016/s0140-6736(20)30734-0 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials