Dose Optimization of Elranatamab to Mitigate the Risk of Cytokine Release Syndrome in Patients with Multiple Myeloma
- PMID: 40000533
- PMCID: PMC11933221
- DOI: 10.1007/s11523-025-01134-8
Dose Optimization of Elranatamab to Mitigate the Risk of Cytokine Release Syndrome in Patients with Multiple Myeloma
Abstract
Background: Elranatamab is a BCMA-CD3 bispecific antibody approved for the treatment of relapsed or refractory multiple myeloma. Cytokine release syndrome is one of the most common adverse events associated with bispecific antibodies.
Objective: We aimed to determine the optimal elranatamab dosing regimen for mitigating cytokine release syndrome.
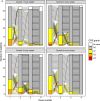
Patients and methods: Safety, pharmacokinetics, and exposure-response were analyzed across four clinical studies (MagnetisMM-1, MagnetisMM-2, MagnetisMM-3, and MagnetisMM-9). Different priming regimens evaluated across these studies included a one-step-up dose priming regimen of 44 mg with or without premedication, a two-step-up dose priming regimen of 12 mg on day 1 and 32 mg on day 4 with premedication, and a two-step-up dose priming regimen of 4 mg on day 1 and 20 mg on day 4 with premedication.
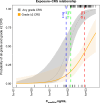
Results: The maximum elranatamab serum concentration on day 1 was positively associated with any-grade and grade ≥ 2 cytokine release syndrome. A slower time to maximum serum concentration and a lower dose-normalized maximum serum concentration were observed with subcutaneous versus intravenous administration, supporting subcutaneous dosing to help mitigate cytokine release syndrome.
Conclusions: Based on the incidence, severity, and predictable profile of cytokine release syndrome, the 12/32-mg priming-dose regimen with premedication was determined to be the optimal regimen before the first full dose of 76 mg on day 8.
Clinical trial registration: ClinicalTrials.gov identifiers: NCT03269136, NCT04798586, NCT04649359, and NCT05014412.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This study was funded by Pfizer. Conflicts of interest/competing interests: Mohamed Elmeliegy, Andrea Viqueira, Erik Vandendries, Anne Hickman, Umberto Conte, Donald Irby, Jennifer Hibma, Hoi-Kei Lon, Joseph Piscitelli, Pooneh Soltantabar, Athanasia Skoura, Sibo Jiang, and Diane Wang report employment and stock ownership at Pfizer. Ethics approval: All studies reported here were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. The study protocols and relevant documents were approved by independent institutional review boards or ethics committees at each investigative center. Consent to participate: All patients provided written informed consent. Consent for publication: Not applicable. Availability of data and material: Upon reasonable request and subject to review, Pfizer will provide the data that support the findings of this article. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizeroncologydevelopment.com/trials for more information. Code availability: Not applicable. Authors’ contributions: All authors were involved in the trial conception/design, or the acquisition, analysis, or interpretation of data. All authors contributed to the drafting of the manuscript and approved the final version.
Figures


References
-
- Costa LJ, Hungria V, Mohty M, Mateos M-V. How I treat triple-class refractory multiple myeloma. Br J Haematol. 2022;198(2):244–56. - PubMed
-
- Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443–8. - PubMed
-
- Pfizer. Elranatamab-bcmm (Elrexfio) [package insert]. New York (NY): Pfizer; 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=19669. Accessed 5 Oct 2023.
-
- European Medicines Agency. Elranatamab-bcmm (Elrexfio) [summary of product characteristics]. 2023. https://www.ema.europa.eu/en/documents/product-information/elrexfio-epar.... Accessed 15 Feb 2024.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

