A phase III study comparing preservative-free latanoprost eye drop emulsion with preserved latanoprost in open-angle glaucoma or ocular hypertension
- PMID: 40000909
- PMCID: PMC12089586
- DOI: 10.1038/s41433-025-03646-z
A phase III study comparing preservative-free latanoprost eye drop emulsion with preserved latanoprost in open-angle glaucoma or ocular hypertension
Abstract
Background/objectives: To evaluate the efficacy and safety of preservative-free latanoprost eye drop emulsion in reducing intraocular pressure (IOP) versus preserved latanoprost in open-angle glaucoma (OAG) or ocular hypertension (OHT).
Methods: A Phase III non-inferiority study randomised patients with OAG/OHT 1:1 to receive preservative-free latanoprost eye drop emulsion or preserved latanoprost. The primary efficacy endpoint was change from baseline in peak (9:00 A.M. ± 1 h) and trough (4:00 P.M. ± 1 h) IOP at Week 12 (non-inferiority margin: 95% confidence interval for treatment difference of ≤1.5 mmHg). Key secondary endpoints were change from baseline in corneal fluorescein staining (CFS) score and in ocular surface disease (OSD) average symptom score at Week 12 (in patients with baseline CFS ≥ 1 or OSD score > 0, respectively).
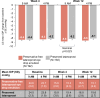
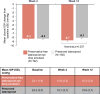
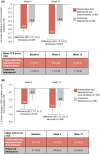
Results: Non-inferiority criteria for IOP-lowering were met. Least square (LS) mean (standard error [SE]) IOP change from baseline with preservative-free latanoprost eye drop emulsion (N = 193) versus preserved latanoprost (N = 193) at Week 12 was -8.8 (0.3) mmHg versus -8.2 (0.3) mmHg at peak (difference: -0.6 mmHg; nominal p = 0.023); -8.6 (0.2) mmHg versus -8.1 (0.3) mmHg at trough (difference: -0.5 mmHg; p = 0.080). LS mean change in CFS (SE) was -0.7 (0.07) with preservative-free latanoprost eye drop emulsion and -0.4 (0.08) with preserved latanoprost (nominal p < 0.001). LS mean change in OSD symptom score was -0.3 (0.1) with preservative-free latanoprost eye drop emulsion and -0.2 (0.1) with preserved latanoprost (nominal p = 0.090).
Conclusions: Preservative-free latanoprost eye drop emulsion demonstrated non-inferior IOP-lowering efficacy compared with preserved latanoprost, and improved signs and symptoms of OSD.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Christophe Baudouin is a consultant for Alcon, Bausch & Lomb, Horus Pharma, Oculis, Santen and Théa. Ingeborg Stalmans received grant/research support from Théa and Santen, clinical trial participation from Théa and Santen, honoraria/consulting fees from Alcon, Allergan/AbbVie, Elios, EyeD, Horus, Santen and Théa, and investments (stock options etc.) from Mona. Rupert Bourne received grant/research support from Théa, Santen, GSK and Allergan/AbbVie. Jose Manuel Larrosa received consulting and speaker fees from Allergan/AbbVie, Bausch & Lomb and Johnson & Johnson. Stefanie Schmickler received honoraria/consulting fees from Bayer, Johnson & Johnson, Kowa, Rayner, and Ziemer and clinical trial fees from Santen. Francesco Oddone is a consultant for AbbVie, Santen, Omikron Italia, Sooft and Théa; received speaker fees from Santen and Omikron Italia, Théa and Aerie; and grants from AbbVie, Santen and Omikron Italia. Aleksey Seleznev has no conflicts of interest to disclose.
Figures
References
-
- European Glaucoma Society. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021;105:1–169. 10.1136/bjophthalmol-2021-egsguidelines. - PubMed
-
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–5. - PubMed
-
- Kolko M, Gazzard G, Baudouin C, Beier S, Brignole-Baudouin F, Cvenkel B, et al. Impact of glaucoma medications on the ocular surface and how ocular surface disease can influence glaucoma treatment. Ocul Surf. 2023;29:456–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

