An antibody-toxin conjugate targeting CD47 linked to the bacterial toxin listeriolysin O for cancer immunotherapy
- PMID: 40000910
- PMCID: PMC11952976
- DOI: 10.1038/s43018-025-00919-0
An antibody-toxin conjugate targeting CD47 linked to the bacterial toxin listeriolysin O for cancer immunotherapy
Abstract
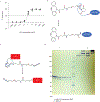
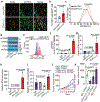
Antigen-presenting cells phagocytose tumor cells and subsequently cross-present tumor-derived antigens. However, these processes are impeded by phagocytosis checkpoints and inefficient cytosolic transport of antigenic peptides from phagolysosomes. Here, using a microbial-inspired strategy, we engineered an antibody-toxin conjugate (ATC) that targets the 'don't eat me' signal CD47 linked to the bacterial toxin listeriolysin O from the intracellular bacterium Listeria monocytogenes via a cleavable linker (CD47-LLO). CD47-LLO promotes cancer cell phagocytosis by macrophages followed by LLO release and activation to form pores on phagolysosomal membranes that enhance antigen cross-presentation of tumor-derived peptides and activate cytosolic immune sensors. CD47-LLO treatment in vivo significantly inhibited the growth of both localized and metastatic breast and melanoma tumors and improved animal survival as a monotherapy or in combination with checkpoint blockade. Together, these results demonstrate that designing ATCs to promote immune recognition of tumor cells represents a promising therapeutic strategy for treating multiple cancers.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: A provisional patent application based on the technology described in the paper has been filed by the Board of Regents, the University of Texas System, with W.J., B.R.S., Y.W. and B.Y.S.K. as inventors. The other authors declare no competing interests.
Figures
















References
-
- Martiniova L, Zielinski RJ, Lin M, DePalatis L & Ravizzini GC The Role of Radiolabeled Monoclonal Antibodies in Cancer Imaging and ADC Treatment. Cancer J 28, 446–453 (2022). - PubMed
-
- Shastry M. et al. Rise of Antibody-Drug Conjugates: The Present and Future. Am Soc Clin Oncol Educ Book 43, e390094 (2023). - PubMed
-
- Motwani M, Pesiridis S & Fitzgerald KA DNA sensing by the cGAS-STING pathway in health and disease. Nat Rev Genet 20, 657–674 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- CTA241186937/Susan G. Komen (Susan G. Komen Breast Cancer Foundation)
- FP00022174/Society for Immunotherapy of Cancer (SITC)
- RR1644/Radiological Society of North America (RSNA)
- PF-24-1156745-01-ET/American Cancer Society (American Cancer Society, Inc.)
- 2024YIA-0832385427/American Society of Clinical Oncology (ASCO)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

