A novel mechanism of FTO modulating the progression of endometriosis through mediating the m6A methylation of GEF-H1 in a YTHDF1-dependent manner
- PMID: 40000966
- PMCID: PMC11863856
- DOI: 10.1186/s10020-025-01130-8
A novel mechanism of FTO modulating the progression of endometriosis through mediating the m6A methylation of GEF-H1 in a YTHDF1-dependent manner
Abstract
Background: Endometriosis (EMs) is a condition characterized by the growth of endometrial tissue outside the uterine cavity. Although this condition is benign, it has cancer-like features. N6-methyladenosine (m6A) is a common RNA modification involved in diverse biological processes, but its role in EMs remains unclear.
Methods: A human endometrial stromal cell line (HESCs), primary eutopic endometrial stromal cells (Eu-ESCs), primary ectopic endometrial stromal cells (Ec-ESCs), and clinical samples were used in this study. A colorimetric assay was used to measure methylation levels in clinical and mouse EMs samples. Functional assays (CCK-8, EdU, Transwell, and wound healing) were used to evaluate phenotypic changes. m6A immunoprecipitation sequencing (MeRIP-seq) identified downstream targets. Mechanistic studies were conducted via qRT‒PCR, Western blot, RNA immunoprecipitation (RIP), dual-luciferase reporter, and RNA stability assays.
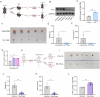
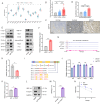
Results: We detected aberrantly low levels of m6A within endometriotic lesions, which was attributed to increased expression of the m6A eraser fat mass and obesity-associated protein (FTO). Notably, estrogen and inflammatory factors, which are recognized as pathogenic agents in EMs amplify FTO expression while suppressing m6A levels. In vitro experiments demonstrated that overexpression of FTO in endometrial stromal cells leads to a reduction in m6A levels and concomitantly promotes their proliferation, migration, and invasion. Furthermore, both genetic deletion of Fto and chemical inhibition of FTO impeded the growth of ectopic endometrial lesions in vivo. By utilizing m6A-seq, we identified GEF-H1 (a Rho guanine nucleotide exchange factor) as a pivotal downstream target of FTO. Specifically, diminished m6A methylation at a certain site within the 3'UTR of GEF-H1 promotes its expression in a YTH N6-methyladenosine RNA-binding protein F1 (YTHDF1)-dependent manner, thereby activating the RhoA pathway. Subsequent experiments revealed that GEF-H1 mediates the effects of FTO in promoting migration and invasion.
Conclusions: This study revealed that FTO decreases the m6A level of GEF-H1, thereby increasing its stability, which in turn activates the GEF-H1-RhoA pathway to promote the migration and invasion of endometrial stromal cells, thereby inducing EMs. Our findings suggest potential therapeutic avenues for targeting FTO to alleviate EMs progression.
Keywords: Endometriosis; Epigenetic modification; FTO; Invasion; Migration; m6A.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Approval was granted by the ethics committee of the First Affiliated Hospital of Xiamen University. Informed consent was obtained from all individual participants included in the study. Ethics approval number: XMYY-2022KYSB012 Consent for publication: We confirm that all the authors have read and approved the final manuscript for submission. Additionally, all authors consent to the publication of the article. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
METTL14 Promotes Proliferation, Migration, and Invasion in Endometriotic Stromal Cell Growth by Activating the ZEB1/MEK/ERK Pathway.Gynecol Obstet Invest. 2025;90(1):42-54. doi: 10.1159/000539656. Epub 2024 Jul 17. Gynecol Obstet Invest. 2025. PMID: 38934184
-
MicroRNA-126-5p downregulates BCAR3 expression to promote cell migration and invasion in endometriosis.Mol Cell Endocrinol. 2019 Aug 20;494:110486. doi: 10.1016/j.mce.2019.110486. Epub 2019 Jun 21. Mol Cell Endocrinol. 2019. PMID: 31233772
-
FTO-dependent N(6)-Methyladenosine regulates the progression of endometriosis via the ATG5/PKM2 Axis.Cell Signal. 2022 Oct;98:110406. doi: 10.1016/j.cellsig.2022.110406. Epub 2022 Jul 14. Cell Signal. 2022. PMID: 35839979
-
Novel Insight of N6-Methyladenosine in Cardiovascular System.Medicina (Kaunas). 2025 Jan 26;61(2):222. doi: 10.3390/medicina61020222. Medicina (Kaunas). 2025. PMID: 40005339 Free PMC article. Review.
-
Recent Advance in Sensitive Detection of Demethylase FTO.Chembiochem. 2025 May 5;26(9):e202400995. doi: 10.1002/cbic.202400995. Epub 2025 Jan 15. Chembiochem. 2025. PMID: 39714929 Review.
Cited by
-
M6A RNA modification: focusing on non-small cell lung cancer progression, therapeutic strategies and challenges.Front Oncol. 2025 Jul 16;15:1622359. doi: 10.3389/fonc.2025.1622359. eCollection 2025. Front Oncol. 2025. PMID: 40740874 Free PMC article. Review.
References
-
- Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666–82. - PubMed
-
- Chen B, Ye F, Yu L, Jia G, Huang X, Zhang X, Peng S, Chen K, Wang M, Gong S, et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134:17963–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

