Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis
- PMID: 40001462
- PMCID: PMC11853291
- DOI: 10.3390/biom15020158
Targeting SMOX Preserves Optic Nerve Myelin, Axonal Integrity, and Visual Function in Multiple Sclerosis
Abstract
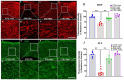
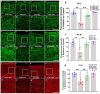
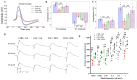
Multiple sclerosis (MS) is a highly disabling chronic neurological condition affecting young adults. Inflammation, demyelination, and axonal damage are key pathological features of MS and its animal model, experimental autoimmune encephalomyelitis (EAE). Our previous work demonstrated that inhibiting spermine oxidase (SMOX) with MDL72527, a selective irreversible pharmacological inhibitor, significantly reduced clinical symptoms, retinal ganglion cell (RGC) loss, and optic nerve inflammation in EAE mice. The present study explored the broader therapeutic potential of SMOX inhibition, focusing on myelin preservation, axonal integrity, and visual function in the EAE model. Electron microscopy of optic nerve cross-sections showed significant preservation of myelin thickness and axonal integrity due to SMOX inhibition. The quantitative assessment showed that g-ratio and axon count metrics were significantly improved in MDL72527-treated EAE mice compared to their vehicle-treated counterparts. Immunofluorescence studies confirmed these findings, showing increased preservation of myelin and axonal proteins in MDL72527-treated EAE mice compared to the vehicle-treated group. Functional assessment studies (Electroretinography) demonstrated significant improvement in RGC function and axonal conduction in EAE mice treated with MDL72527. Furthermore, SMOX inhibition downregulated the expression of galectin3 (Gal3), a mediator of neuroinflammation, indicating Gal3's role in SMOX-mediated neuroprotection. This study provides compelling evidence for the potential of SMOX inhibition as a therapeutic strategy in multiple sclerosis and other demyelinating disorders.
Keywords: MDL72527; multiple sclerosis; myelin; optic nerve; spermine oxidase; vision.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Treatment with MDL 72527 Ameliorated Clinical Symptoms, Retinal Ganglion Cell Loss, Optic Nerve Inflammation, and Improved Visual Acuity in an Experimental Model of Multiple Sclerosis.Cells. 2022 Dec 16;11(24):4100. doi: 10.3390/cells11244100. Cells. 2022. PMID: 36552864 Free PMC article.
-
HE3286 reduces axonal loss and preserves retinal ganglion cell function in experimental optic neuritis.Invest Ophthalmol Vis Sci. 2014 Aug 19;55(9):5744-51. doi: 10.1167/iovs.14-14672. Invest Ophthalmol Vis Sci. 2014. PMID: 25139738 Free PMC article.
-
Limiting Neuronal Nogo Receptor 1 Signaling during Experimental Autoimmune Encephalomyelitis Preserves Axonal Transport and Abrogates Inflammatory Demyelination.J Neurosci. 2019 Jul 10;39(28):5562-5580. doi: 10.1523/JNEUROSCI.1760-18.2019. Epub 2019 May 6. J Neurosci. 2019. PMID: 31061088 Free PMC article.
-
Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects.J Steroid Biochem Mol Biol. 2016 Jun;160:43-52. doi: 10.1016/j.jsbmb.2016.01.006. Epub 2016 Jan 14. J Steroid Biochem Mol Biol. 2016. PMID: 26776441 Free PMC article. Review.
-
MOG35 - 55-induced EAE model of optic nerve inflammation compared to MS, MOGAD and NMOSD related subtypes of human optic neuritis.J Neuroinflammation. 2025 Apr 7;22(1):102. doi: 10.1186/s12974-025-03424-4. J Neuroinflammation. 2025. PMID: 40197321 Free PMC article. Review.
References
-
- Qian Z., Li Y., Guan Z., Guo P., Zheng K., Du Y., Yin S., Chen B., Wang H., Jiang J., et al. Global, regional, and national burden of multiple sclerosis from 1990 to 2019: Findings of global burden of disease study 2019. Front. Public Health. 2023;11:1073278. doi: 10.3389/fpubh.2023.1073278. - DOI - PMC - PubMed
-
- Mike A., Glanz B.I., Hildenbrand P., Meier D., Bolden K., Liguori M., Dell’Oglio E., Healy B.C., Bakshi R., Guttmann C.R. Identification and clinical impact of multiple sclerosis cortical lesions as assessed by routine 3T MR imaging. AJNR Am. J. Neuroradiol. 2011;32:515–521. doi: 10.3174/ajnr.A2340. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

