APOL1 Dynamics in Diabetic Kidney Disease and Hypertension
- PMID: 40001508
- PMCID: PMC11853202
- DOI: 10.3390/biom15020205
APOL1 Dynamics in Diabetic Kidney Disease and Hypertension
Abstract
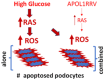
APOL1 Renal Risk Variants (APOL1RRVs, G1, and G2) are known to be toxic to glomerular podocytes and causally associated with an enhanced prevalence and progression of many different etiologies of chronic kidney disease (CKD), leading to the delineation of a new disease designation of APOL1-Mediated Kidney Disease (AMKD). Notably, APOL1RRVs have not consistently been shown to increase the prevalence or severity of diabetic kidney disease (DKD) progression, which is the most common cause of End-Stage Kidney Disease (ESKD). While this apparent discrepancy seems perplexing, its clarification should provide important mechanistic and therapeutic insights. Activation of the Renin-Angiotensin System (RAS) plays a critical role in the development and progression of DKD. Recent in vitro and in vivo studies also demonstrated that RAS activation contributes to kidney cell injury in AMKD experimental models. Both high glucose, as well as APOL1RRVs escalate the podocyte expression of miR193a, a known mediator of glomerulosclerosis, including idiopathic Focal Segmental Glomerular Sclerosis (FSGS) and DKD. We propose that either the RAS and/or miR193a levels in the diabetic milieu are already maximally conducive to kidney target cell injury and, therefore, are agnostic to further injury in response to APOL1RRVs. Similarly, the contributory role of hypertension (which is frequently reported as the second most common cause of ESKD) in the progression of AMKD remains a controversial issue. Since several clinical reports have shown that controlling hypertension does not consistently slow the progression of AMKD, this has led to a formulation wherein APOL1-RRVs primarily lead to kidney injury with accompanying hypertension. Notably, half a decade later, the notion that hypertension is not a cause but rather a consequence of kidney injury was contested by investigators analyzing the Mount Sinai BioMe repository, a comprehensive clinical and genetic database including participants with APOL1RRVs. These investigators observed that hypertension predated the observed decline in GFR in individuals with APOL1RRVs by ten years. In the present study, we discuss the mechanistic forces that may underpin the gaps in these clinical manifestations, which did not allow the temporal association of hypertension with AMKD to be translated into causation and may also dissociate DKD and AMKD. We have hypothesized models that need to be validated in future experimental studies.
Keywords: APOL1; chronic kidney disease; diabetic kidney disease; hypertension; miR193a; renin–angiotensin system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Genovese G., Friedman D.J., Ross M.D., Lecordier L., Uzureau P., Freedman B.I., Bowden D.W., Langefeld C.D., Oleksyk T.K., Knob A.L.U., et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. - DOI - PMC - PubMed
-
- Tzur S., Rosset S., Shemer R., Yudkovsky G., Selig S., Tarekegn A., Bekele E., Bradman N., Wasser W.G., Behar D.M., et al. Missense mutations in the APOL1 gene are highly associated with end-stage kidney disease risk previously attributed to the MYH9 gene. Hum. Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. - DOI - PMC - PubMed
-
- Fine D.M., Wasser W.G., Estrella M.M., Atta M.G., Kuperman M., Shemer R., Rajasekaran A., Tzur S., Racusen L.C., Skorecki K. APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J. Am. Soc. Nephrol. 2012;23:343–350. doi: 10.1681/ASN.2011060562. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

