Meliponini Geopropolis Extracts Induce ROS Production and Death in Leishmania amazonensis Promastigotes and Axenic Amastigotes In Vitro
- PMID: 40001930
- PMCID: PMC11851448
- DOI: 10.3390/biology14020162
Meliponini Geopropolis Extracts Induce ROS Production and Death in Leishmania amazonensis Promastigotes and Axenic Amastigotes In Vitro
Abstract
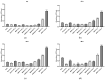
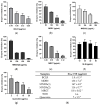
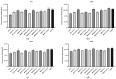
Leishmania amazonensis, a cause of cutaneous leishmaniasis in Brazil, is a neglected disease with toxic and inconsistently effective treatments. The parasite's survival depends on managing oxidative stress, making redox-regulating enzymes potential therapeutic targets. Geopropolis, a resinous product from native stingless bees, shows promising antiparasitic effects. This study aims to evaluate the anti-L. amazonensis activity of geopropolis produced by Melipona bicolor, M. marginara, M. mondury, and M. quadrifasciata (two samples), targeting enzymes responsible for the parasite's redox balance. Ethanol extracts of geopropolis produced by each bee (BCRL, MRGT, MNDY, MNDA(1), and MNDA(2), respectively) were analyzed for total phenolics and flavonoids. Promastigotes and axenic amastigotes were treated with various extract concentrations, and parasite viability was assessed using the resazurin reduction method. Cytotoxicity was tested on peritoneal macrophages, RAW 264.7, VERO cell lines (MTT assay), and erythrocytes (hemolysis assay). Additionally, mitochondrial dehydrogenase activity, reactive oxygen species (ROS) production, the inhibition of recombinant arginase, and autophagic activity were also evaluated in treated parasites. MRGT showed the highest levels of phenolics (762 mg GAE/g) and flavonoids (345 mg QE/g). MDRY was more effective against promastigote and axenic amastigote forms (IC50 = 168 and 19.7 µg/mL, respectively). MRGT showed lower cytotoxicity against RAW 264.7 and VERO (CC50 = 654 µg/mL and 981 µg/mL, respectively). Erythrocytes exhibited reduced sensitivity to MNDA(2) (HC50 = 710 µg/mL). The activity of dehydrogenases and LiARG was reduced by treating the parasites with the extracts following the induction of ROS and autophagic activity. These results highlight geopropolis extracts as a source of substances with anti-L. amazonensis activity capable of inducing oxidative stress on the parasite.
Keywords: human cutaneous leishmaniasis; leishmanicidal activity; mitochondrial activity; native bee products.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- WHO W.H.O. Leishmaniasis. [(accessed on 10 October 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
-
- Benicio E.D.A., Nunes Gadelha E.P., Talhari A., Silva R.M.D., Jr., Ferreira L.C., Santos M.C.C.D., Mira M.T., Oliveira C.M.C.D., Talhari C., Talhari S., et al. Combining Diagnostic Procedures for the Management of Leishmaniasis in Areas with High Prevalence of Leishmania Guyanensis. An. Bras. Dermatol. 2011;86:1141–1144. doi: 10.1590/S0365-05962011000600012. - DOI - PubMed
-
- Carvalho S.H., Frézard F., Pereira N.P., Moura A.S., Ramos L.M.Q.C., Carvalho G.B., Rocha M.O.C. American Tegumentary Leishmaniasis in Brazil: A Critical Review of the Current Therapeutic Approach with Systemic Meglumine Antimoniate and Short-term Possibilities for an Alternative Treatment. Trop. Med. Int. Health. 2019;24:380–391. doi: 10.1111/tmi.13210. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

