Aggressive Serous Carcinomas of the Female Reproductive Tract: Cancer-Prone Cell States and Genetic Drivers
- PMID: 40002199
- PMCID: PMC11852459
- DOI: 10.3390/cancers17040604
Aggressive Serous Carcinomas of the Female Reproductive Tract: Cancer-Prone Cell States and Genetic Drivers
Abstract
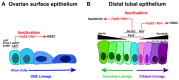
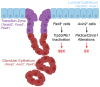
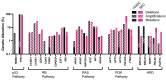
In 2025, gynecological cancers are projected to account for approximately 10% of cancer-related deaths in women. High-grade serous ovarian carcinoma (HGSC) and serous endometrial carcinoma (SEC) are the most lethal gynecological cancer subtypes. Both malignancies commonly have TP53 mutations, alterations of the RB1 pathway, and numerous secondary mutations. Both carcinoma types consist of poorly differentiated and highly heterogeneous cell populations at the time of detection. Latent development and rapid progression of HGSC and SEC impede the identification of definitive cells of origin and genetic drivers. Here, we review our current knowledge about cancer-prone cell states and genetic drivers. We also discuss how emerging transcriptomic and genetic tools applied to contemporary model systems may facilitate the identification of novel targets for timely detection and therapeutic intervention.
Keywords: cancer-prone cell state; genetic drivers; ovarian cancer; uterine cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma: A Rereview of Cases Lacking TP53 Mutations in The Cancer Genome Atlas Ovarian Study.Int J Gynecol Pathol. 2016 Jan;35(1):48-55. doi: 10.1097/PGP.0000000000000207. Int J Gynecol Pathol. 2016. PMID: 26166714 Free PMC article.
-
Early Genetic Divergence of High-Grade Carcinomas Originating from Low-Grade Serous Ovarian Neoplasms.Mod Pathol. 2025 Jan;38(1):100629. doi: 10.1016/j.modpat.2024.100629. Epub 2024 Oct 9. Mod Pathol. 2025. PMID: 39389422
-
Precursors and pathogenesis of ovarian carcinoma.Pathology. 2013 Apr;45(3):229-42. doi: 10.1097/PAT.0b013e32835f2264. Pathology. 2013. PMID: 23478230 Review.
-
Evidence for lineage continuity between early serous proliferations (ESPs) in the Fallopian tube and disseminated high-grade serous carcinomas.J Pathol. 2018 Nov;246(3):344-351. doi: 10.1002/path.5145. Epub 2018 Sep 27. J Pathol. 2018. PMID: 30043522
-
Cell Origins of High-Grade Serous Ovarian Cancer.Cancers (Basel). 2018 Nov 12;10(11):433. doi: 10.3390/cancers10110433. Cancers (Basel). 2018. PMID: 30424539 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

