Triple-Negative Breast Cancer Systemic Treatment: Disruptive Early-Stage Developments for Overcoming Stagnation in the Advanced Pipeline
- PMID: 40002228
- PMCID: PMC11853049
- DOI: 10.3390/cancers17040633
Triple-Negative Breast Cancer Systemic Treatment: Disruptive Early-Stage Developments for Overcoming Stagnation in the Advanced Pipeline
Abstract
New breast cancer (BC) diagnoses will soon reach 2.5-3 million/year worldwide, with 15-25% of them being triple-negative breast cancer (TNBC), the most aggressive type, characterized for lacking the main pharmacological targets: estrogen and progesterone receptors (ERs and PRs), as well as HER2 overexpression. Therefore, chemotherapy remains the almost-unique systemic treatment for TNBC. However, some targeted therapies are recommended for use in combination with chemotherapy; namely, PARP inhibitors for BRCA-mutated TNBC, the immune checkpoint inhibitors pembrolizumab and atezolizumab, as well as the antibody-drug conjugates sacituzumab govitecan and trastuzumab deruxtecan, the latter for HER2low subtypes. Regardless of the limited benefits they provide, other treatments with similar mechanisms of action are being investigated in advanced clinical stages. Further, therapies that benefit other cancers, like PI3K/Akt/mTOR pathway and CDK4/6 inhibitors, are still being investigated for TNBC, although convincing results have not been obtained. Given this scenario, it might appear innovation for TNBC treatments has become stuck. However, the huge unmet medical need drives intense research into the biology of the disease. As a result, emerging disruptive therapies are being tested in early-stage trials, designed for novel targets and applying cutting-edge advances in immunotherapy and precision oncology.
Keywords: monoclonal antibody; onco-immunotherapy; small molecule inhibitor; systemic treatment; targeted therapy; triple-negative breast cancer.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Eva González-Suárez:
Figures
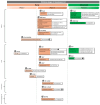
 . Additionally, the
. Additionally, the  label is used for those studies with an unknown status. Targets of each mAb are indicated and labeled with the symbol
label is used for those studies with an unknown status. Targets of each mAb are indicated and labeled with the symbol  . Left panel specifies the type of mAb: “ADC”, antibody–drug conjugate; “ICI”, immune checkpoint inhibitor; “chimeric” refers to molecules that, although built on them, cannot be considered mAbs sensu stricto.
. Left panel specifies the type of mAb: “ADC”, antibody–drug conjugate; “ICI”, immune checkpoint inhibitor; “chimeric” refers to molecules that, although built on them, cannot be considered mAbs sensu stricto.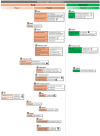
 , or terminated, labeled with
, or terminated, labeled with  , CTs at the highest phase are shown instead. Additionally, the
, CTs at the highest phase are shown instead. Additionally, the  label is used for those studies with an unknown status. Targets of each molecule are indicated and labeled with the symbol
label is used for those studies with an unknown status. Targets of each molecule are indicated and labeled with the symbol  .
.References
-
- Wolff A.C., Hammond M.E.H., Hicks D.G., Dowsett M., McShane L.M., Allison K.H., Allred D.C., Bartlett J.M., Bilous M., Fitzgibbons P., et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. - DOI - PubMed
-
- Lehmann B.D., Jovanović B., Chen X., Estrada M.V., Johnson K.N., Shyr Y., Moses H.L., Sanders M.E., Pietenpol J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

