Targeting Kinesins for Therapeutic Exploitation of Chromosomal Instability in Lung Cancer
- PMID: 40002279
- PMCID: PMC11853690
- DOI: 10.3390/cancers17040685
Targeting Kinesins for Therapeutic Exploitation of Chromosomal Instability in Lung Cancer
Abstract
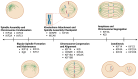
New therapeutic approaches that antagonize tumour-promoting phenotypes in lung cancer are needed to improve patient outcomes. Chromosomal instability (CIN) is a hallmark of lung cancer characterized by the ongoing acquisition of genetic alterations that include the gain and loss of whole chromosomes or segments of chromosomes as well as chromosomal rearrangements during cell division. Although it provides genetic diversity that fuels tumour evolution and enables the acquisition of aggressive phenotypes like immune evasion, metastasis, and drug resistance, too much CIN can be lethal because it creates genetic imbalances that disrupt essential genes and induce severe proteotoxic and metabolic stress. As such, sustaining advantageous levels of CIN that are compatible with survival is a fine balance in cancer cells, and potentiating CIN to levels that exceed a tolerable threshold is a promising treatment strategy for inherently unstable tumours like lung cancer. Kinesins are a superfamily of motor proteins with many members having functions in mitosis that are critical for the correct segregation of chromosomes and, consequently, maintaining genomic integrity. Accordingly, inhibition of such kinesins has been shown to exacerbate CIN. Therefore, inhibiting mitotic kinesins represents a promising strategy for amplifying CIN to lethal levels in vulnerable cancer cells. In this review, we describe the concept of CIN as a therapeutic vulnerability and comprehensively summarize studies reporting the clinical and functional relevance of kinesins in lung cancer, with the goal of outlining how kinesin inhibition, or "targeting kinesins", holds great potential as an effective strategy for treating lung cancer.
Keywords: chromosomal instability; kinesin; lung cancer; targeted therapy.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Targeting the Mitotic Kinesin KIF18A in Chromosomally Unstable Cancers: Hit Optimization Toward an In Vivo Chemical Probe.J Med Chem. 2022 Mar 24;65(6):4972-4990. doi: 10.1021/acs.jmedchem.1c02030. Epub 2022 Mar 14. J Med Chem. 2022. PMID: 35286090
-
Quantifying chromosomal instability from intratumoral karyotype diversity using agent-based modeling and Bayesian inference.Elife. 2022 Apr 5;11:e69799. doi: 10.7554/eLife.69799. Elife. 2022. PMID: 35380536 Free PMC article.
-
Small-molecule inhibition of kinesin KIF18A reveals a mitotic vulnerability enriched in chromosomally unstable cancers.Nat Cancer. 2024 Jan;5(1):66-84. doi: 10.1038/s43018-023-00699-5. Epub 2023 Dec 27. Nat Cancer. 2024. PMID: 38151625 Free PMC article.
-
The yin and yang of chromosomal instability in prostate cancer.Nat Rev Urol. 2024 Jun;21(6):357-372. doi: 10.1038/s41585-023-00845-9. Epub 2024 Feb 2. Nat Rev Urol. 2024. PMID: 38307951 Free PMC article. Review.
-
Role of chromosomal instability in cancer progression.Endocr Relat Cancer. 2017 Sep;24(9):T23-T31. doi: 10.1530/ERC-17-0187. Epub 2017 Jul 10. Endocr Relat Cancer. 2017. PMID: 28696210 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

