Do Lifestyle Interventions Mitigate the Oxidative Damage and Inflammation Induced by Obesity in the Testis?
- PMID: 40002337
- PMCID: PMC11851673
- DOI: 10.3390/antiox14020150
Do Lifestyle Interventions Mitigate the Oxidative Damage and Inflammation Induced by Obesity in the Testis?
Abstract
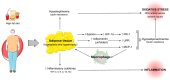
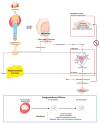
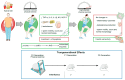
Obesity results from a disproportionate accumulation of fat and has become a global health concern. The increase in adipose tissue is responsible for several systemic and testicular changes including hormone levels (leptin, adiponectin, testosterone, estrogen), inflammatory cytokines (increase in TNF-α and IL-6 and decrease in IL-10), and redox state (increase in reactive oxygen species and reduction in antioxidant enzymes). This results in poor sperm quality and compromised fertility in men with obesity. Lifestyle modifications, particularly diet transition to caloric restriction and physical exercise, are reported to reverse these negative effects. Nevertheless, precise mechanisms mediating these benefits, including how they modulate testicular oxidative stress, inflammation, and metabolism, remain to be fully elucidated. The main pathway described by which these lifestyle interventions reverse obesity-induced oxidative damage is the Nrf2-SIRT1 axis, which modulates the overexpression of antioxidant defenses. Of note, some of the detrimental effects of obesity on the testis are inherited by the descendants of individuals with obesity, and while caloric restriction reverses some of these effects, no significant work has been carried out regarding physical exercise. This review discusses the consequences of obesity-induced testicular oxidative stress on adult and pediatric populations, emphasizing the therapeutic potential of lifestyle to mitigate these detrimental effects.
Keywords: caloric restriction; inflammation; obesity; oxidative stress; physical exercise; sperm parameters; testosterone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Ameliorating high-fat diet-induced sperm and testicular oxidative damage by micronutrient-based antioxidant intervention in rats.Eur J Nutr. 2022 Oct;61(7):3741-3753. doi: 10.1007/s00394-022-02917-9. Epub 2022 Jun 16. Eur J Nutr. 2022. PMID: 35708759 Free PMC article.
-
Effect of Different Exercise Loads on Testicular Oxidative Stress and Reproductive Function in Obese Male Mice.Oxid Med Cell Longev. 2020 Jan 27;2020:3071658. doi: 10.1155/2020/3071658. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32082477 Free PMC article.
-
A high-fat, high-protein diet attenuates the negative impact of casein-induced chronic inflammation on testicular steroidogenesis and sperm parameters in adult mice.Gen Comp Endocrinol. 2017 Oct 1;252:48-59. doi: 10.1016/j.ygcen.2017.07.013. Epub 2017 Jul 22. Gen Comp Endocrinol. 2017. PMID: 28743557
-
Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans.Int J Obes (Lond). 2006 Mar;30(3):400-18. doi: 10.1038/sj.ijo.0803177. Int J Obes (Lond). 2006. PMID: 16302012 Review.
-
Towards a comprehensive theory of obesity and a healthy diet: The causal role of oxidative stress in food addiction and obesity.Behav Brain Res. 2020 Apr 20;384:112560. doi: 10.1016/j.bbr.2020.112560. Epub 2020 Feb 17. Behav Brain Res. 2020. PMID: 32081711 Review.
Cited by
-
The microRNA-mediated apoptotic signaling axis in male reproduction: a possible and targetable culprit in male infertility.Cell Biol Toxicol. 2025 Mar 5;41(1):54. doi: 10.1007/s10565-025-10006-w. Cell Biol Toxicol. 2025. PMID: 40038116 Free PMC article. Review.
References
-
- World Health Organization . World Health Statistics 2024: Monitoring Health for the SDGs, Sustainable Development Goals. World Health Organization; Geneva, Switzerland: 2024.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

