Osteosarcoma Cells and Undifferentiated Human Mesenchymal Stromal Cells Are More Susceptible to Ferroptosis than Differentiated Human Mesenchymal Stromal Cells
- PMID: 40002376
- PMCID: PMC11852062
- DOI: 10.3390/antiox14020189
Osteosarcoma Cells and Undifferentiated Human Mesenchymal Stromal Cells Are More Susceptible to Ferroptosis than Differentiated Human Mesenchymal Stromal Cells
Abstract
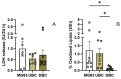
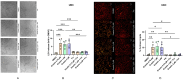
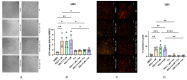
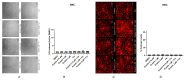
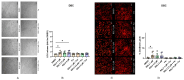
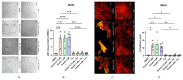
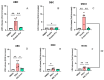
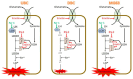
Current research suggests that promoting ferroptosis, a non-apoptotic form of cell death, may be an effective therapy for osteosarcoma, while its inhibition could facilitate bone regeneration and prevent osteoporosis. Our objective was to investigate whether the susceptibility to and regulation of ferroptosis differ between undifferentiated (UBC) and differentiated (DBC) human bone marrow stromal cells, as well as human osteosarcoma cells (MG63). Ferroptosis was induced by either inhibiting glutathione peroxidase 4 (GPX4) using RSL3 or blocking all glutathione-dependent enzymes through inhibition of the glutamate/cysteine antiporter with Erastin. Lipid peroxidation was assessed using the fluorescent probe BODIPY™581/591C11, while Ferrostatin-1 was used to inhibit ferroptosis. We demonstrate that neither Erastin nor RSL3 induces ferroptosis in DBC. However, both RSL3 and Erastin induce ferroptosis in UBC, while Erastin predominantly induces ferroptosis in MG63 cells. Our data suggest that ferroptosis induction in undifferentiated hBMSCs is primarily regulated by GPX4, whereas glutathione S-Transferase P1 (GSTP1) plays a key role in controlling ferroptosis in osteosarcoma cells. In conclusion, targeting the key pathways involved in ferroptosis across different bone cell types may improve the efficacy of cancer treatments while minimizing collateral damage and supporting regenerative processes, with minimal impact on cancer therapy.
Keywords: Erastin; Ferrostatin-1; RSL3; ferroptosis; osteosarcoma.
Conflict of interest statement
The authors declare no conflicts of interest. Austrian Cluster for Tissue Regeneration is a non-profit association, which does not have either employees or the budget. All funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures



References
-
- Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

