Supplementation with the Postbiotic BPL1™-HT (Heat-Inactivated Bifidobacterium animalis subsp. Lactis) Attenuates the Cardiovascular Alterations Induced by Angiotensin II Infusion in Mice
- PMID: 40002381
- PMCID: PMC11851978
- DOI: 10.3390/antiox14020193
Supplementation with the Postbiotic BPL1™-HT (Heat-Inactivated Bifidobacterium animalis subsp. Lactis) Attenuates the Cardiovascular Alterations Induced by Angiotensin II Infusion in Mice
Abstract
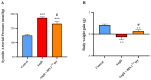
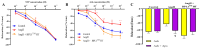
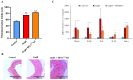
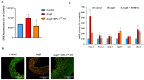
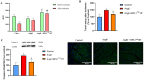
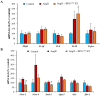
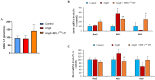
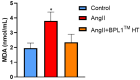
Hypertension is associated with alterations in the composition and diversity of the intestinal microbiota. Indeed, supplementation with probiotics and prebiotics has shown promising results in modulating the gut microbiota and improving cardiovascular health. However, there are no studies regarding the possible beneficial effects of postbiotics on cardiovascular function and particularly on hypertension-induced cardiovascular alterations. Thus, the aim of this study was to analyze the effect of supplementation with the heat-treated Bifidobacterium animalis subsp. lactis CECT 8145 strain (BPL1™ HT), a postbiotic developed by the company ADM-Biopolis, on cardiovascular alterations induced by angiotensin II (AngII) infusion in mice. For this purpose, three groups of C57BL/6J male mice were used: (i) mice infused with saline (control); (ii) mice infused with AngII for 4 weeks (AngII); and (iii) mice supplemented with BPL1™ HT in the drinking water (1010 cells/animal/day) for 8 weeks and infused with AngII for the last 4 weeks (AngII + BPL1™ HT). AngII infusion was associated with heart hypertrophy, hypertension, endothelial dysfunction, and overexpression of proinflammatory cytokines in aortic tissue. BPL1™ HT supplementation reduced systolic blood pressure and attenuated AngII-induced endothelial dysfunction in aortic segments. Moreover, mice supplemented with BPL1™ HT showed a decreased gene expression of the proinflammatory cytokine interleukin 6 (Il-6) and the prooxidant enzymes NADPH oxidases 1 (Nox-1) and 4 (Nox-4), as well as an overexpression of AngII receptor 2 (At2r) and interleukin 10 (Il-10) in arterial tissue. In the heart, BPL1™ HT supplementation increased myocardial contractility and prevented ischemia-reperfusion-induced cardiomyocyte apoptosis. In conclusion, supplementation with the postbiotic BPL1™ HT prevents endothelial dysfunction, lowers blood pressure, and has cardioprotective effects in an experimental model of hypertension induced by AngII infusion in mice.
Keywords: angiotensin II; antioxidant; cardiovascular damage; hypertension; inflammation; postbiotic.
Conflict of interest statement
Since this work was carried out in collaboration with the company ADM Wild/Biopolis, authors from this company may have a conflict of interest. These authors have participated in the production and characterization of the postbiotic BPL1™ HT. The in vivo study was performed by the academic researchers from Universidad Autónoma de Madrid who do not have conflict of interests.
Figures
References
-
- Fyhrquist F., Metsarinne K., Tikkanen I. Role of angiotensin II in blood pressure regulation and in the pathophysiology of cardiovascular disorders. J. Hum. Hypertens. 1995;9((Suppl. 5)):S19–S24. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

