Emergent Aspects of the Integration of Sensory and Motor Functions
- PMID: 40002495
- PMCID: PMC11853489
- DOI: 10.3390/brainsci15020162
Emergent Aspects of the Integration of Sensory and Motor Functions
Abstract
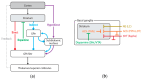
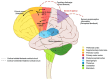
This article delves into the intricate mechanisms underlying sensory integration in the executive control of movement, encompassing ideomotor activity, predictive capabilities, and motor control systems. It examines the interplay between motor and sensory functions, highlighting the role of the cortical and subcortical regions of the central nervous system in enhancing environmental interaction. The acquisition of motor skills, procedural memory, and the representation of actions in the brain are discussed emphasizing the significance of mental imagery and training in motor function. The development of this aspect of sensorimotor integration control can help to advance our understanding of the interactions between executive motor control, cortical mechanisms, and consciousness. Bridging theoretical insights with practical applications, it sets the stage for future innovations in clinical rehabilitation, assistive technology, and education. The ongoing exploration of these domains promises to uncover new pathways for enhancing human capability and well-being.
Keywords: imagery; motor control; rehabilitation; sensorimotor integration.
Conflict of interest statement
The author declares no conflicts of interest.
Figures

References
-
- Turecek J., Ginty D. How two intermingled sensory pathways combine to encode touch. Nature. 2022. online ahead of print . - PubMed
Publication types
LinkOut - more resources
Full Text Sources

