Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies
- PMID: 40002740
- PMCID: PMC11852430
- DOI: 10.3390/biomedicines13020327
Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies
Abstract
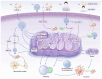
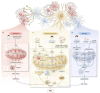
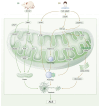
Neurodegenerative disease (ND) refers to the progressive loss and morphological abnormalities of neurons in the central nervous system (CNS) or peripheral nervous system (PNS). Examples of neurodegenerative diseases include Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS). Recent studies have shown that mitochondria play a broad role in cell signaling, immune response, and metabolic regulation. For example, mitochondrial dysfunction is closely associated with the onset and progression of a variety of diseases, including ND, cardiovascular diseases, diabetes, and cancer. The dysfunction of energy metabolism, imbalance of mitochondrial dynamics, or abnormal mitophagy can lead to the imbalance of mitochondrial homeostasis, which can induce pathological reactions such as oxidative stress, apoptosis, and inflammation, damage the nervous system, and participate in the occurrence and development of degenerative nervous system diseases such as AD, PD, and ALS. In this paper, the latest research progress of this subject is detailed. The mechanisms of oxidative stress, mitochondrial homeostasis, and mitophagy-mediated ND are reviewed from the perspectives of β-amyloid (Aβ) accumulation, dopamine neuron damage, and superoxide dismutase 1 (SOD1) mutation. Based on the mechanism research, new ideas and methods for the treatment and prevention of ND are proposed.
Keywords: mitochondrial dynamics; mitochondrial energy metabolism; mitochondrial homeostasis imbalance; mitophagy; neurodegenerative diseases.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Brody J.A., Stanhope J.M., Kurland L.T. Patterns of Amyotrophic Lateral Sclerosis and Parkinsonism-Dementia on Guam. Contemp. Neurol. Ser. 1975;12:45–70. - PubMed
-
- Messner D.A., Rabins P., Downing A.C., Irizarry M., Foster N.L., Al Naber J., Dabbous O., Fillit H., Gabler S., Krakauer R., et al. Designing Trials of Disease Modifying Agents for Early and Preclinical Alzheimer’s Disease Intervention: What Evidence is Meaningful to Patients, Providers, and Payers? J. Prev. Alzheimers Dis. 2019;6:20–26. doi: 10.14283/jpad.2018.42. - DOI - PubMed
Publication types
Grants and funding
- 600791001/the Research Start-up Fund of Jining Medical University
- JYHL2021MS13/Research Fund for Lin He's Academician Workstation of New Medicine and Clinical Translation in Jining Medical University
- 81700055/the National Natural Science Foundation of China
- Grant No. D2016021/Outstanding Talent Research Funding of Xuzhou Medical University
- BK20160229/Natural Science Foundation of Jiangsu Province
LinkOut - more resources
Full Text Sources
Miscellaneous

