Metabolic Reprogramming in Gut Microbiota Exposed to Polystyrene Microplastics
- PMID: 40002859
- PMCID: PMC11853289
- DOI: 10.3390/biomedicines13020446
Metabolic Reprogramming in Gut Microbiota Exposed to Polystyrene Microplastics
Abstract
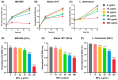
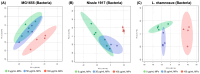
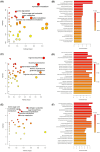
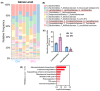
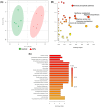
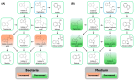
Background: Microplastics (MPs) are small plastic fragments with diameters less than 5 mm in size and are prevalent in everyday essentials and consumables. Large global plastic production has now led to a flooding of MPs in our natural environment. Due to their detrimental impacts on the planet's ecosystems and potentially our health, MPs have emerged as a significant public health concern. In this pilot study, we hypothesize that MPs exposure will negatively affect gut microbiota composition and function, in which metabolic reprogramming plays an important role. Methods: Using in vitro experiments, three bacterial strains (Escherichia coli MG1655, Nissle 1917, and Lactobacillus rhamnosus) were selected to investigate the impacts of MPs exposure. The bacterial strains were individually cultured in an anaerobic chamber and exposed to 1 µm polystyrene MPs at various concentrations (0, 10, 20, 50, 100, and 500 µg/mL) in the culture medium. Results: MPs exposure reduced the growth of all three bacterial strains in a dose-dependent manner. Liquid chromatography mass spectrometry (LC-MS)-based untargeted metabolomics revealed significant differences in multiple metabolic pathways, such as sulfur metabolism and amino sugar and nucleotide sugar metabolism. In addition, we extracted gut microbiota from C57BL/6 mice, and 16S rRNA sequencing results showed a significant upregulation of Lactobacillales and a significant reduction in Erysipelotrichales due to MPs exposure. Furthermore, targeted and untargeted metabolomics corroborated the in vitro results and revealed alterations in microbial tryptophan metabolism and energy producing pathways, such as glycolysis/gluconeogenesis and the pentose phosphate pathway. Conclusions: These findings provide evidence that MPs exposure causes comprehensive changes to healthy gut microbiota, which may also provide insights into the mechanistic effects of MPs exposure in humans.
Keywords: 16S rRNA; gut microbiota; mass spectrometry; metabolomics; microplastics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

